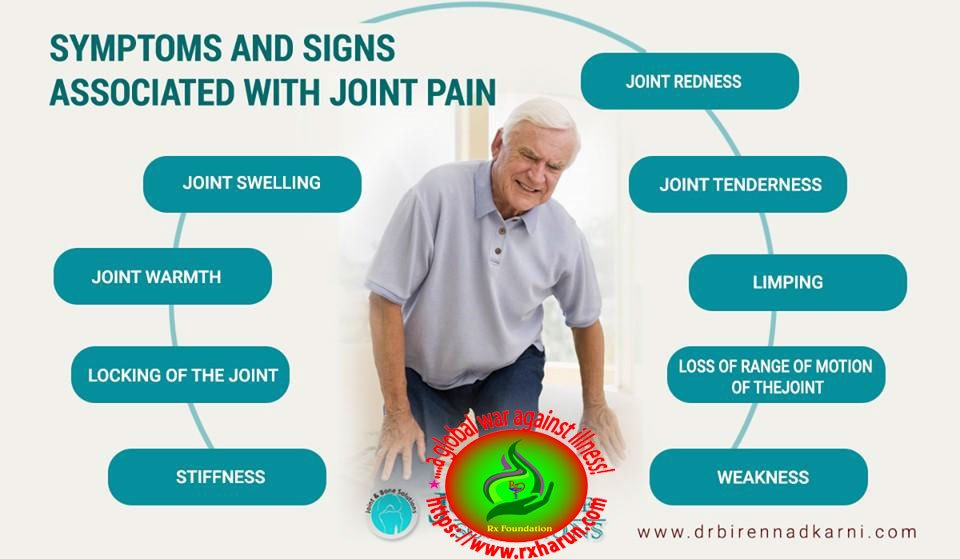
Joint Aspiration/ Arthrocentesis is the clinical procedure of using a syringe to collect synovial fluid from a joint capsule. It is also known as joint aspiration. Arthrocentesis is used in the diagnosis of gout, arthritis, and synovial infections such as septic arthritis.
Arthrocentesis is a procedure performed to aspirate synovial fluid from a joint cavity. It is used both diagnostically and therapeutically. While arthrocentesis is deemed to be a minor surgical procedure, there is always the potential to injure blood vessels, nerves, and tendons. Thus, the procedure should only be performed by healthcare workers who do have knowledge of the anatomy of joints. To minimize the risk of injury, the extensor surface of the joint should be in extension with minimal flexion.[rx][rx]
Why is arthrocentesis performed?
Your doctor may recommend an arthrocentesis to diagnose and possibly treat the following diseases and conditions of the joints:
- Arthritis – or inflammation of the joint. Both osteoarthritis and rheumatoid arthritis can lead to pain, swelling and destruction of the joint.
- Autoimmune disorders – such as lupus, which often leads to joint pain and swelling in some cases
- Bleeding into the joint – (hemarthrosis), such as bleeding caused by a torn ligament or a bleeding disorder
- Crystal-induced arthritis – such as gout
- Cysts – filled with synovial fluid. Cysts can form as a complication of arthritis and breakdown of the joint.
- Injury to the joint – such as a fracture or a torn ligament or cartilage
- Joint infection – which is also called septic arthritis
- Unexplained joint effusion – which is a buildup of synovial fluid with swelling of the joint. Arthrocentesis is used to remove the excess fluid.
Indications of Arthrocentesis / Joint Aspiration
Indications for diagnostic knee arthrocentesis include the following:
-
Evaluation of monoarticular arthritis
-
Evaluation of suspected septic arthritis
-
Evaluation of joint effusion
-
Identification of intra-articular fracture
-
Identification of crystal arthropathy
Indications for therapeutic knee arthrocentesis include the following:
-
Relief of pain by aspirating effusion or blood
-
Injection of medications (eg, corticosteroids, antibiotics, or anesthetics)
-
Drainage of septic effusion
Contra-Indications of Arthrocentesis / Joint Aspiration
There are no absolute contraindications for knee arthrocentesis. Relative contraindications include the following
Absolute
- Periarticular or overlying skin and soft tissue infection in the area of the joint or a systemic infection due to the risk of seeding joint with infection.
- Corticosteroid or local anesthetic injection are contraindicated in the presence of a documented allergy to the medications planned for injection (N.B. amide anesthetics display cross-reactivity).
Relative
- Disrupted or denuded skin, especially if chronic (e.g., in dermatoses such as psoriasis, venous stasis ulcers) due to the risk of chronic bacterial colonization.
- Anatomic disruption (dislocation, fracture, tendon tear or rupture, prosthetic joint).
Considerations
- If the patient is on full strength anticoagulation, consideration of risks – benefits should be given. There is evidence that joint aspiration/injection is safe in patients on warfarin with INR up to 4.5.
- If the patient has severe immunosuppression, consideration of risks – benefits should be given relative to the indication (e.g., a strong indication for arthrocentesis in concern for septic joint versus low urgency for arthrocentesis and corticosteroid injection for chronic DJD with a small effusion).
-
Cellulitis overlying the joint – If arthrocentesis is performed, the patient should be admitted for the administration of intravenous (IV) antibiotics, even if the synovial fluid is not suggestive of infectious arthritis
-
Skin lesion or dermatitis overlying the joint
-
Known bacteremia
-
Adjacent osteomyelitis
-
Uncontrolled coagulopathy
-
Joint prosthesis – Preferably, a joint prosthesis is tapped by an orthopedist
| Contents of Arthrocentesis Tray |
|---|
|
|
|
|
|
|
|
|
| Syringes |
|
|
|
|
| Tubes |
|
|
|
|
|
|
|
|
|
Precautions: Occupational Safety and Health Administration guidelines state
| Table 4: Comparison of Commonly Used Intraarticular Corticosteroids Preparations | |||||
| Trade Name | Generic Name | Concentration (mg/mL) | Equivalent Doses | The range of Water Solubility | Dosing (mg/mL) |
|---|---|---|---|---|---|
| Depo-Medrol | Methylprednisolone acetate | 20, 40, 80 | 4 | Insoluble | 10-80 |
| Aristospan | Triamcinolone hexacetonide | 20 | 4 | Insoluble | 5-40 |
| Kenalog, Aristocort | Triamcinolone acetonide | 20 | 4 | Soluble | 5-40 |
| Celestone | Betamethasone acetate | 6 | 0.6 | Insoluble | 1.5-6 |
| Hydeltra | Prednisolone tebutate | 20 | 5 | Soluble | 5-50 |
| Table 5: Materials and Doses for Joint Injections | |||||
| Joint | Needle Length (gauge)* | Volume of Intraarticular Injection (mL) | Dose of Depo-Medrol (mg) | ||
|---|---|---|---|---|---|
| Knee | 1.5 in. (22/18) | 1-3 | 40-80 | ||
| Shoulder | 1.5 in. (22/18 or 19) | 1-3 | 40-80 | ||
| Wrist | 1 – 1.5 in. (22/19) | 0.5-2 | 20-40 | ||
| Ankle | 1.5 in. (22/19) | 0.5-2 | 20-40 | ||
| Elbow | 1.5 in. (22./18) | 0.5-2 | 20-40 | ||
| MCP | 5/8-1 in. (25/21) | 0.25-0.5 | 5-10 | ||
| PIP | 5/8-1 in. (25/23) | 0.25-0.5 | 5-10 | ||
| MTP | 5/8-1 in. (25/21) | 0.25-0.5 | 5-10 | ||
| *Sizes suggested apply to needle gauge during installation only or aspiration/installation, respectively. | |||||
Supplies needed for arthrocentesis and/or joint injection
- Gloves (clean, non-sterile gloves may be used. The injection site should not be touched after cleaning/sterilizing. This is called “no touch” technique and is supported by multiple published studies as a best practice).
- Iodine swabs x 3 or chlorhexidine swab
- Gauze pads
- Needles
- 18 gauge for larger joint aspiration, 23 gauge for smaller joint aspiration
- 25 gauge for injection only including pre-arthrocentesis local anesthetic injection
- Length: 2” needle for medium and large joints, 1-1.5” for small joints
- Syringes: 5 mL+ for aspiration, for injection use size appropriate for the volume
- Adhesive bandage/dressing
- Ethyl chloride spray or topical anesthetic ointment for skin anesthesia (if needed)
- Corticosteroid (if needed)
- 1% or 2% lidocaine without epinephrine or another local anesthetic (if needed)
- Ultrasound (if needed)
A brief guide to injectable medications
| Joint size (examples) | Triamcinolone acetonide dose* (vol of 40 mg/1mL) | Hydrocortisone equivalent (if converting to other steroid)** | 1% Lidocaine volume in mL (dose in mg)*** |
|
Large
|
40 mg (1mL)
|
200 mg
|
4-10 mL (50-100 mg)
|
|
Medium
|
10-20 mg (0.25-0.5 mL)
|
50-100 mg
|
3-5 mL (30-50 mg)
|
|
Small
|
1-10 mg (0.05-0.25 mL)
|
5-50 mg
|
1-3 mL (10-30 mg)
|
For a brief guide to injectable medications.
Laboratory testing
For most effusions without clear diagnosis, send the following:
- Cell count and differential
- Protein and glucose
- Bacterial culture and gram stain
- Microscopy (e.g., polarized light microscopy in suspected gout or pseudogout)
- Consider testing for acid-fast bacillus (AFB) and fungus if clinically indicated
Technique
The clinician or medical care professional must obtain informed consent. A timeout should be performed to confirm patient and correct joint. The most important step is having the patient lay in a comfortable position with the affected knee fully extended or flexed at 15 to 20 degrees with a towel roll under the knee. This position helps to facilitate procedure success by ensuring quadriceps muscle relaxation. The clinician should then locate the patella. The clinician may mark it with a marking pen.
The knee is the largest synovial cavity in the body and can easily be accessed from either the medial or lateral aspect, and superior, inferior at the midpoint of the patella.
Sterilize area and drape in typical sterile fashion.
Choose the approach, then use a small syringe and small bore needle, draw up lidocaine and anesthetize superficial skin and then deeper tissue in the projected trajectory of joint aspiration to anesthetize the track.
-
For midpoint approach, insert 18 g needle with 30 cc to 60 cc syringe one cm lateral or medial to the patella, directing the needle posterior and horizontal toward the intercondylar notch of the femur.
-
Make sure to pull back on the syringe while inserting and stop once you aspirate synovial fluid.
-
Attempt to aspirate as much fluid as possible.
-
The superior approach is performed one cm superior and one cm medial or lateral to the patella, directing towards intercondylar notch of the femur.
-
The infrapatellar approach requires the patient to be sitting upright, with the knee flexed at 90 degrees. Needle insertion is five mm below the inferior border of patella while also directing posterior to the patellar tendon, making this a less desirable approach.
“Milking” or compressing the joint can help facilitate aspiration of fluid.
Transfer fluid to specimen tubes. Remove the needle from joint and place bandage over the insertion site.
PERFORMING THE PROCEDURE
Step 1: Preparation
- For arthrocentesis only, use an 18 gauge (medium and large joints) or 22 gauge needle (small joints) connected to a 5-60 cc syringe depending on the size of the joint and volume of fluid needed. 5-10 cc is likely to be adequate for routine testing. For therapeutic arthrocentesis, a larger syringe may be indicated.
- For arthrocentesis followed by corticosteroid injection, use needle and syringe above for the arthrocentesis and prepare a separate 5-10 cc syringe for medications (e.g., for the knee: a 5 cc syringe containing 1 cc of 40 mg/mL triamcinolone acetonide mixed with 4 cc of plain lidocaine).
- For corticosteroid injection use 25 gauge needle with steroid mixture in a syringe prepared as above.
Step 2: Anesthetize the area (optional depending on preference and procedure)
Skin anesthesia/analgesia can be accomplished in multiple methods. For arthrocentesis with a large needle, skin anesthesia may be preferable, whereas it may be unnecessary if performing a joint injection with a 25 gauge needle. Discuss options with patient in advance:
- Ice
- Stretching skin (counter-traction which limits discomfort from skin distention)
- Ethyl chloride spray
- Topical anesthetic (e.g., EMLA) administration
- Cutaneous anesthesia with 1-3 mL lidocaine and a 25G needle
If using ice, ethyl chloride or topical anesthetics, apply these prior to skin prep. If using lidocaine injection for local anesthesia (e.g., prior to large needle arthrocentesis), inject ~1-3ml lidocaine with a 25 gauge needle subcutaneously at the marked site. Wait a few minutes following the injection of lidocaine for anesthetic effect prior to arthrocentesis.
Step 3: Aspirate / Inject
For arthrocentesis, apply counter-traction to the skin outside the sterilized area and enter the skin with needle at the marked site, advancing toward the joint space while pulling back syringe plunger with gentle consistent force. You will create a small vacuum. When you enter the joint, the syringe will begin to fill with synovial fluid.
Once fluid enters the syringe, stop advancing the needle and try to maintain position for the rest of the aspiration. Once in the joint space, aspirate synovial fluid to the volume needed, based on intended studies or need for therapeutic drainage.
If the needle moves and/or flow stops prior to the desired volume, try to advance and retract it slightly, without radically repositioning it.
If no aspirate is found, pull the needle back until you are in the soft tissue, but not out of the skin. Quickly reassess what direction is optimal to reach the joint space. Redirect the needle right or left, superiorly or inferiorly, depending on your goal location. Advance again, until the joint fluid is found.
If you are performing arthrocentesis followed by joint injection (sometimes called “two syringes, one needle technique”), hold the hub firmly between the thumb and index finger, with the little finger/ulnar aspect of the hand resting against the patient’s skin for stability. Unscrew the syringe and exchange syringes for the syringe containing medications for injection. Once the new syringe is in place, inject the medication. If resistance is felt, you may need to retract or change position slightly if the needle bore is obstructed.
If performing corticosteroid and/or local anesthetic intra-articular injection alone, apply counter-traction to the skin outside the sterilized area and enter the skin with a needle at the marked site, advance until you are in the joint space (depth depending on the size and depth of the joint and size of the patient.) Inject all of the medication at the desired depth. Avoid injecting into tendons (this is usually noted as resistance to injection, retract until medication flows freely in this case) and avoid injection into the dermis (injection of steroids can cause dermal fat atrophy.)
When aspiration and/or injection are completed, remove the needle, apply pressure with sterile gauze, wipe away iodine with alcohol and apply an adhesive bandage.
Landmarks
A series of photos and brief descriptions of approaches to arthrocentesis and joint injection for common injections: shoulder glenohumeral joint and subacromial (extra-articular) space, elbow, knee, ankle tibiotalar joint, and 1st MTP joint.
Post-injection education
- Decrease to normal level of activity (no strenuous activity) for a few days following corticosteroid injection, following by a gradual return to usual activity.
- Discuss signs and symptoms of infection and precautions – return to primary care, urgent care or hospital emergency department immediately.
- Discuss signs of post-injection flare in patients receiving corticosteroid injection and plan of care – ice, NSAID’s/APAP, relative rest.
- In case of joint injection with corticosteroid and local anesthetic, patients may experience immediate relief from anesthetic for hours. This effect will wane, usually followed by the slower onset of pain relief in 48 hours as the steroid begins to take effect.
- Stretching/strengthening/resting as indicated based on the underlying diagnosis.
Interpretation of synovial fluid analysis (adapted from Bettencourt and Linder, 2010)
| Normal | Non-inflammatory(e.g., DJD/OA) | Inflammatory(e.g., autoimmune, crystal disease) | Septic | |
|
Total WBC/mm3
|
<100
|
100-2,000
|
2,000-50,000
|
>25,000-50,000
|
|
PMN (%)
|
<25
|
<25
|
<50
|
>50-90
|
|
Culture
|
Negative
|
Negative
|
Negative
|
Often positive
|
for Interpretation of synovial fluid analysis.
What are the possible complications of arthrocentesis and/or joint injection?
Arthrocentesis and joint injection
- Iatrogenic infection (1/10000 to 1/100,000) – this generally takes around 12-48 hrs to develop) – requires urgent referral to orthopedics for joint lavage
Corticosteroid/local anesthetic injection only
- Post-injection flare in 1-6%, though due to the inflammatory response to corticosteroid crystals (generally begins 2 hours-2 days after injection with corticosteroids) – self-limited over 3-5 days and responds to NSAID’s)
- Impaired glucose control (for approximately 1-2 weeks in patients with pre-existing diabetes or impaired glucose tolerance. Hyperglycemia occurs in a dose-responsive manner to potency and amount of corticosteroid used)
- Tendon rupture
- Fat necrosis/calcification
Preparation for Aspiration/Injection
Materials for aseptic skin preparation
- Sterile gloves.
- Iodine solution.
- Alcohol solution.
- Sterile gauze pads.
Materials for local anesthesia
- One percent lidocaine for skin, subcutaneous tissues, and joint structures.
- Ethyl chloride spray for skin.
Needles – Sterile 18- to 25-gauge needles, depending on the size of the joint. Inflamed joint fluids may be thick and require a large-bore needle for removal. Sterile 30-gauge needles may be used for local anesthetic instillation, and for injection into small joints such as the proximal interphalangeal joints and when no fluid aspiration is anticipated.
- Syringes, 1cc to 50cc in size, depending on the joint and amount of effusion.
Tubes for synovial fluid analysis
- Hematology tube for cell count and differential.
- Sterile tubes for Gram stain, cultures and smears.
- Heparinized tube for crystal analysis. Powdered anticoagulant may interfere with crystal identification.
- Cytology bottle (if neoplasm is suspected).
Intra-articular medications – At Hospital for Special Surgery, methylprednisolone acetate (Depomedrol), a long-acting, insoluble corticosteroid preparation is used. The dose varies with the size of the joint. Betamethasone (Celestone) may be used when the goal is avoidance of a joint flare reaction and a shorter 2-4 week duration of action is acceptable (e.g. with attacks of crystal disease) Doses and appropriate needle sizes are summarized in Table 8-1.

Table 8-1 Intra-articular Therapy Regimens
Joint-Specific Techniques
The most important maneuver before aspirating a joint is to locate the appropriate landmark. This can be best done by making a skin impression with the round end of a non-opened pen point or pen mark. Local anesthesia of the overlying skin and subcutaneous tissues is recommended. In general, if it is important to obtain fluid for diagnostic purposes, a larger needle should be used (so as to avoid the need for re-aspiration if the fluid is too thick to be aspirated by a small needle).
Shoulder – The shoulder can be entered either anteriorly or posteriorly.
- Anterior approach – (Fig. 8-1). With the patient’s hand in the lap and the shoulder muscles relaxed, the glenohumeral joint can be palpated by placing the fingers between the coracoid process and the humeral head. As the shoulder is internally rotated, the humeral head can be felt turning inward and the joint space can be felt as a groove just lateral to the coracoid process. When the skin over this area is anesthetized, a 20- or 22-gauge needle can be inserted lateral to the coracoid. (the thoracoacromial artery lies medial to the coracoid). The needle is directed dorsally and medially into the joint space. The needle should be directed slightly superiorly to avoid the neurovascular bundle.

Arthrocentesis of the shoulder – anterior approach.
Posterior approach (Fig. 8-2). The posterior aspect of the shoulder joint is identified with the patient’s arm internally rotated maximally. This position is achieved by placing the patient’s ipsilateral hand on the opposite shoulder. The humeral head can then be palpated by placing a finger posteriorly along the acromion while the shoulder is rotated. A 20- or 22-gauge needle is inserted about 1 cm inferior to the posterior tip of the acromion and directed anteriorly and medially.

Figure 8-2. Arthrocentesis of the shoulder – posterior approach.
Elbow – The elbow joint (Fig. 8-3) can be identified by placing the patient’s relaxed arm in the lap. With the palm facing the patient, flex the elbow to a 45-degree angle. Place your finger on the lateral epicondyle and note the shallow depression distal to it, which represents the elbow joint. A 22-gauge needle is introduced perpendicular to the joint.

Figure 8-3. Arthrocentesis of the elbow.
Wrist – (Fig. 8-4) aspiration is performed on the dorsal aspect just distal to the radius or ulna as indicated by clinical examination.
- Radial entry – The hand and wrist are relaxed in a slightly flexed position. The joint space can be located by palpating the edge of the distal radius just medial to the thumb extensor tendon. A 22-gauge needle should be directed into the joint from the dorsal aspect.
- Ulnar entry – Keep the wrist in the same relaxed position. The joint space can be identified by palpating just distal to the distal ulna. The 22-gauge needle is directed in a volar and radial direction.

Figure 8-4. Arthrocentesis of the wrist – medial and lateral approaches.
Ankle – For both approaches (Fig. 8-5), the foot is first placed at about a 45-degree angle of plantar flexion.
- Medial approach – A 22-gauge needle is placed about 1 in. proximal and lateral to the distal end of the medial malleolus. The flexor hallucis longus tendon is just lateral to this point. The needle is directed 45 degrees posteriorly, slightly upward, and laterally.
- Lateral approach – A 22-gauge needle is placed about 1/2 in. proximal and medial to the distal end of the lateral malleolus. The needle should be directed 45 degrees posteriorly, slightly upward, and medially.

Figure 8-5. Arthrocentesis of the ankle – medial and lateral approaches.
Knee – The knee (Fig. 8-6) is the easiest joint to enter. It may be entered either medially or laterally. The patient should be supine with the knee comfortably extended. A 19-22-gauge needle is introduced in a direction parallel to the plane of the posterior surface of the patella in a medial position (in patients with a known coagulopathy, or taking anticoagulant drugs, a 25g needle can be used). With thick exudative effusions, a larger-bore needle may be required. Drainage of the knee suprapatellar bursa can be facilitated by compressing the suprapatellar pouch during aspiration. With large knee effusions, the distended suprapatellar pouch can be aspirated directly from either the medial or lateral aspect of the quadriceps muscle mass.

Figure 8-6. Arthrocentesis of the knee – medial approach
Small joints of the hands and feet – may be difficult to enter. Occasionally, the effusion bulges and facilitates aspiration. Often, a corticosteroid injection can be performed just adjacent to the joint rather than within it; this may result in an equivalent clinical response.
- The metacarpophalangeal (MCP) joint – can be easily palpated on its dorsal, lateral aspect with the finger slightly flexed and relaxed. The joint is entered on the dorsal-lateral aspect with a 22 to 25-gauge needle. Because this is a ball (distal metacarpal) and cup (first phalanx) joint, the needle should not be directed at a 90-degree angle but rather distally at about a 60-degree angle.
- The proximal interphalangeal (PIP) joint – margin is barely palpable but may be felt on its dorsal aspect just distal to the skin crease. The joint is entered from the dorsal aspect with a 25 to the 30-gauge needle that is directed slightly distally.
- The distal interphalangeal (DIP) joint – is extremely small and difficult to enter. The technique is the same as for aspirating the PIP joint. If there is no suspicion of infection, a 30g needle may be the least uncomfortable for the patient and easiest for joint entry.
- The metatarsophalangeal (MTP) joint – is aspirated in a fashion similar to that for the MCP joint.
- Other joints – There are external landmarks that can direct aspiration and injection of the hip joint, but success in this venture requires some experience. Because the hip joint lies deep within the pelvis, aspiration can be more readily performed under fluoroscopic or ultrasound guidance. Ultrasound, in particular, enables direct visualization, accurately defines the presence and size of effusion and allows for successful and safe aspiration to be performed if need be. The injection of corticosteroids for treatment can also be done with greater precision in administration. The spinal and sacroiliac joints often demand fluoroscopic or CT guidance.
Complications of Arthrocentesis
- Post-injection flare – Long-acting intra-articular steroid preparations like methylprednisolone acetate may induce crystal synovitis 24 hours after the injection. Application of ice at the onset of a post injection flare may be helpful
- Bruising – The needle itself may traumatize the joint, especially if the joint is small; for this reason, the patient should be warned of possible short-term aggravation of symptoms in the injected joint and receive appropriate instructions regarding analgesia
- Skin atrophy – can sometimes occur if corticosteroids are injected close to the undersurface of the skin.
- Tendon rupture – Injection of corticosteroids directly into a tendon or tendon insertion can cause rupture. If tendon injection is desired, ultrasound-guided injections directly into the paratenon (not the tendon proper) may be both effective and safe. Ultrasound-guided injections, e.g. into the retrocalcaneal bursa, can minimize the risk of injection into the Achilles tendon itself.
Synovial Fluid Analysis
Fluid analysis is an extremely useful diagnostic tool in the evaluation of rheumatic diseases. It should be included in the initial evaluation of most arthritic conditions that generate effusions. It can yield a specific diagnosis in infectious and crystal-induced arthritis and can be helpful in categorization, definition and the diagnosis of other arthritides.
Synovial fluid studies
- Gross examination – can be helpful in establishing the nature of joint fluid. After air bubbles are allowed to clear, a heparinized specimen is examined for the following:
- Color – Normal synovial fluid is straw-colored. Inflammatory fluids range from yellow to greenish yellow. Bloody fluid to one degree or another occurs in patients with coagulation disorders, trauma, neoplasms, and tuberculous arthritis and in patients receiving anticoagulant therapy.
- Clarity – Normal synovial fluids are clear enough that print can be read through them. As inflammation increases from mild to marked, the fluid becomes first translucent and then opalescent.
- Viscosity – Synovial fluid viscosity is tested by allowing a drop of fluid to fall from the needle tip. Normal synovial fluids are quite viscous, and a “string” of fluid will form. Because viscosity is decreased in inflammatory synovial fluids, no string sign is seen.
- Cell Count – A cell count for both white blood cells (WBC), including a differential, and red blood cells (RBC) should be performed. Inflammatory fluids generally have WBC counts in the thousands, while non-inflammatory fluids have WBC counts under 1000. Remember the ratio of RBC/WBC is ~750/1. This can be important in hemorrhagic fluids that are inflammatory or infected. The WBC differential can be useful diagnostically, with greater than 50% polymorphonuclear (PMN) cells common in inflammatory effusions and greater than 90% PMN cells commonly seen with infection.
- Crystal examination – can be performed using polarized microscopy of a specimen of heparinized fluid (see Chapters 43, 44). Urate crystals parallel to the polarizer axis appear yellow, and urate crystals perpendicular to the polarizer axis appear blue. The opposite is true for the calcium pyrophosphate crystals of pseudogout. Urate crystals are needle-shaped and calcium pyrophosphate crystals are rhomboid. Remember that the finding of crystals does not rule out the possibility of an infection.
Microbiologic studies
- Stains – should include both Gram’s and acid-fast methods if infection is suspected or fluid appears turbid or cloudy.
- Cultures – should include routine bacterial studies. Fungal and mycobacterial cultures are ordered if clinically indicated. Some fastidious or slower-growing organisms may need to be watched for growth for up to 4 weeks. Synovial fluids suspicious for gonococci or Borrelia burgdorferi may be specifically sent for PCR analysis if the diagnosis is in question.
Biochemical studies
- Glucose – Determination of synovial fluid glucose, when interpreted with a simultaneous serum value, is helpful in diagnosing infectious arthritis. In bacterial infection or tuberculosis, the synovial fluid glucose will be less than half the serum value. Occasionally, low values may be seen in RA.
- Protein – determination does not provide additional useful information and should not be routinely ordered.
- Complement – may be decreased in RA, but the test is rarely helpful for diagnosis because synovial fluid complement is usually normal in early RA.
Diagnosis by fluid group –Synovial fluid can be divided into three groups based on the degree of inflammation
- Group 1 fluids – are clear and transparent and have few white cells on cell count. They include normal, osteoarthritis, and systemic lupus erythematosus (SLE) joint fluids.
- Group 2 fluids – generally have a higher WBC count and are not as reactive as group 1 fluids; they appear translucent. This group includes fluids from most non-infectious, inflammatory arthritic conditions such as gout, pseudogout, psoriatic arthritis, Reactive arthritis and RA. Leukemia or lymphoma occasionally presents in this category, but the differential count reveals more than 90% mononuclear cells.
- Group 3 fluids – are opalescent or purulent. Group 3 fluids include those from bacterial infections and tuberculosis (although joint fluid from gonococcal arthritis can be either group 2 or group 3). Group 3 fluids typically have 50,000 to 300,000 WBC per milliliter; these are mostly neutrophils. Occasionally, the synovial fluid from a patient with an inflammatory arthritic condition such as RA or gout may have as many as 50,000 to 75,000 white cells per milliliter and appears opalescent or even purulent. As Table 8-1 shows, there is considerable overlap between the various arthritic diseases; this table is meant to serve as a guideline rather than provide a rigid set of criteria.
References
[bg_collapse view=”button-orange” color=”#4a4949″ expand_text=”Show More” collapse_text=”Show Less” ]
- https://www.ncbi.nlm.nih.gov/books/NBK470229/
- http://www.medicinenet.com/joint_aspiration/article.htm
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2218651/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3844254/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2213980/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3953519/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3577627/
- https://www.ncbi.nlm.nih.gov/pubmed/21050951
- https://www.ncbi.nlm.nih.gov/pubmed/19250236
[/bg_collapse]