Diabetic retinopathy (DR) is the most common complication of diabetes and remains a major cause of preventable blindness. Anatomical and functional changes occur in various retinal cells including retinal endothelial cells, neurons, and retinal pigment epithelium prior to clinical symptoms of the disease. Early changes include the appearance of microaneurysms, leukocyte adhesion, apoptosis of vascular (endothelial cells and pericytes), and neuronal cells.
Diabetic retinopathy is a potentially blinding complication of diabetes mellitus. Reasons for loss of vision are diabetic maculopathy and complications of proliferative diabetic retinopathy (PDR) such as vitreous hemorrhage, tractional retinal detachment, and neovascular glaucoma.
Diabetic retinopathy is the most common microvascular complication of diabetes mellitus and affects between 3%-4% of people in Europe, while the relative risk for developing diabetic retinopathy is higher in type 1 diabetes compared to type 2[rx–rx]. Diabetes mellitus is responsible for about 15% of all cases of legal blindness (best corrected visual acuity less than 0.02) in Germany[rx]. It is the main cause of blindness within the working-age population in industrialized nations[rx].
Types of Diabetic Retinopathy
1. According to the clinical condition
Clinically, DR is divided into two stages
- Non-proliferative diabetic retinopathy (NPDR)
- Proliferative diabetic retinopathy (PDR).
- NPDR represents the early stage of DR, wherein increased vascular permeability and capillary occlusion are two main observations in the retinal vasculature. PDR, a more advanced stage of DR, is characterized by neovascularization. During this stage, the patients may experience severe vision impairment when the new abnormal vessels bleed into the vitreous (vitreous hemorrhage) or when tractional retinal detachment is present. The most common cause of vision loss in patients with DR is diabetic macular edema (DME). DME is characterized by swelling or thickening of the macula due to sub- and intra-retinal accumulation of fluid in the macula triggered by the breakdown of the blood-retinal barrier (BRB) [rx].
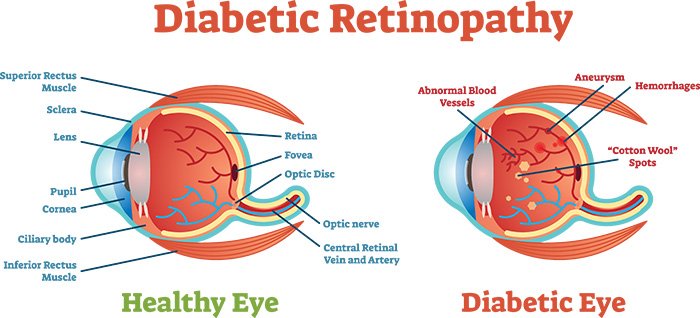
Non-proliferative and proliferative diabetic retinopathy
Non-proliferative diabetic retinopathy (NPDR) is characterized by the presence of
- (i) microaneurysms, which are the first clinically detectable lesions of DR located in the inner nuclear layer of the retina,
- (ii) dot and blot hemorrhages, which are located in the middle retinal layers,
- (iii) hard exudates, which are located between the inner plexiform and inner nuclear layer of the retina,
- (iv) vascular changes such as beading, looping, and sausage like segmentation of the veins,
- (v) cotton wool spots also called soft exudates or nerve fiber infarcts, result from capillary occlusion of the retinal nerve fiber layer,
- (vi) intraretinal microvascular abnormalities (IRMA), which are dilated capillaries that seem to function as collateral channels, frequently seen adjacent to the areas of capillary closure,
- (vii) retinal edema characterized by the accumulation of fluid between the outer plexiform layer and inner nuclear layer, which may later involve the entire layers of the retina.
Background Retinopathy
- Tiny bulges develop in the blood vessels, which may bleed slightly but don’t usually affect your vision
Pre-Proliferative Retinopathy
- More severe and widespread changes affect the blood vessels, including more significant bleeding into the eye
Clinical classification is as follows
Mild Non-Proliferative Diabetic Retinopathy
- At least one microaneurysm, and also a dot, blot or flame-shaped hemorrhages in all four fundus quadrants.
Moderate Non-Proliferative Diabetic Retinopathy
- Intraretinal microaneurysms and dot and blot hemorrhages of greater severity, in one to three quadrants. Cotton wool spots, venous caliber changes including venous beading, and intraretinal microvascular abnormalities are present but mild.
Severe Non-Proliferative Diabetic Retinopathy
- At least one of the following should be present: a) ‘severe’ hemorrhages and microaneurysms in all four quadrants of the fundus, b) venous beading, which is more marked in at least two quadrants, and c) intraretinal microvascular abnormalities, which are more severe in at least one quadrant.
Very Severe Non-Proliferative Diabetic Retinopathy
- Two or more of the criteria for severe non-proliferative diabetic retinopathy, but without any proliferative diabetic retinopathy.
According to Symptom or infection time
There are two types of diabetic retinopathy
- Early diabetic retinopathy – In this more common form — called nonproliferative diabetic retinopathy (NPDR) — new blood vessels aren’t growing (proliferating).
When you have NPDR, the walls of the blood vessels in your retina weaken. Tiny bulges (microaneurysms) protrude from the vessel walls of the smaller vessels, sometimes leaking fluid and blood into the retina. Larger retinal vessels can begin to dilate and become irregular in diameter, as well. NPDR can progress from mild to severe, as more blood vessels become blocked. Nerve fibers in the retina may begin to swell. Sometimes the central part of the retina (macula) begins to swell (macular edema), a condition that requires treatment.
- Advanced diabetic retinopathy – Diabetic retinopathy can progress to this more severe type, known as proliferative diabetic retinopathy. In this type, damaged blood vessels close off, causing the growth of new, abnormal blood vessels in the retina, and can leak into the clear, jelly-like substance that fills the center of your eye (vitreous).
Causes of Diabetic Retinopathy
Factors that turn the diabetic retinopathy
- There are a number of growth factors which have been associated with the development of diabetic retinopathy and these include basic fibroblast growth factor (bFGF) [rx], insulin-like growth factor-1 (IGF-1) [rx, rx], angiopoietin-1 and -2 stromal-derived factor-1 [rx], epidermal growth factor (EGF) [rx], transforming growth factor-beta 2 (TGF-β2) [rx], platelet-derived growth factors (PDGFs) [rx], and erythropoietin [rx].
- The insulin-like growth factors (IGFs) are produced by the majority of body tissues and are mediators of cell growth, differentiation, and transformation. Increased levels of IGF-1 have been found in the vitreous fluid and serum of diabetic patients [rx, rx]. The precise role of IGF in diabetic retinopathy pathogenesis remains unknown. However, increasing evidence suggests that IGFs work directly within target tissues as well as systemically [rx, rx].
Criteria and degree of urgency for referral of a patient with DR to the ophthalmologist.
| Lesions requiring immediate assessment by the ophthalmologist | Proliferative retinopathy | (i) New vessels on the optic disc or at any location in the retina |
| (ii) Preretinal hemorrhage | ||
| Advanced diabetic retinopathy | (i) Vitreous hemorrhage | |
| (ii) Fibrotic tissue (epiretinal membrane) | ||
| (iii) Recent retinal detachment | ||
| (iv) Iris neovascularization | ||
| Lesions that should be referred to the ophthalmologist for assessment as soon as possible | Preproliferative retinopathy | (i) Venous irregularities |
| (ii) Multiple hemorrhages | ||
| (iii) Multiple cotton-wool exudates | ||
| (iv) Intraretinal microvascular abnormalities (IRMA) | ||
| Nonproliferative retinopathy with macular involvement | (i) Decreased visual acuity uncorrected with a pinhole occluder (suggestive of macular edema) | |
| (ii) Microaneurysms, hemorrhages, or exudates within less than one disc diameter of the center of the macula (with or without vision loss) | ||
| Nonproliferative retinopathy without macular involvement | (i) Hard exudates with a circinate or plaque pattern in the major temporal vascular arcades | |
| Any other finding that the observer could not be interpreted with a reasonable degree of certainty | ||
| Lesions requiring follow-up control (every 6–12 months) but should not be referred to the ophthalmologist | Nonproliferative retinopathy | (i) Hemorrhages or microaneurysms occasionally or hard exudates beyond one disc diameter of the center of the macula |
| (ii) Isolated cotton-wool exudates without preproliferative associated lesions | ||
Symptoms of Diabetic Retinopathy
Many people often do not have symptoms until very late in their disease course. Patients often become symptomatic when there is irreversible damage.[rx] Symptoms are usually not painful and can include
- Seeing spots or floaters
- Blurred vision
- Having a dark or empty spot in the center of your vision
- Difficulty seeing well at night
- Gradually worsening vision
- Sudden vision loss
- Shapes floating in your field of vision (floaters)
- Eye pain or redness
- Vitreous hemorrhage
- Floaters, or small objects that drift through the field of vision
- Decreased visual acuity
- “Curtain falling” over eyes
Diagnosis of Diabetic Retinopathy
Diabetic retinopathy can be diagnosed through a comprehensive eye examination. Testing, with emphasis on evaluating the retina and macula, may include:
- Patient history – to determine vision difficulties, the presence of diabetes, and other general health concerns that may be affecting vision
- Refraction to determine if a new eyeglass prescription is needed
- Evaluation of the ocular structures, including the evaluation of the retina through a dilated pupil
- Measurement of the pressure within the eye
Diabetic retinopathy and DME are detected during a comprehensive dilated eye exam that includes:
- Visual acuity testing – This eye chart test measures a person’s ability to see at various distances.
- Tonometry – This test measures pressure inside the eye.
- Pupil dilation – Drops placed on the eye’s surface dilate (widen) the pupil, allowing a physician to examine the retina and optic nerve.
- Optical coherence tomography (OCT) – This technique is similar to ultrasound but uses light waves instead of sound waves to capture images of tissues inside the body. OCT provides detailed images of tissues that can be penetrated by light, such as the eye.
A comprehensive dilated eye exam allows the doctor to check the retina for
- Changes to blood vessels
- Leaking blood vessels or warning signs of leaky blood vessels, such as fatty deposits
- Swelling of the macula (DME)
- Changes in the lens
- Damage to nerve tissue
Supplemental testing may include
- Retinal photography or tomography to document the current status of the retina
- Fluorescein angiography to evaluate abnormal blood vessel growth
Insulin-dependent/juvenile-onset diabetes
-
Dilated fundus examination every year beginning 5 years after diagnosis, from puberty onwards
-
Examinations more frequently once diabetic retinopathy is diagnosed.
Non insulin-dependent/maturity-onset diabetes
-
Dilated fundus examination every year once diabetes diagnosed
-
Examination more frequently once diabetic retinopathy diagnosed.
Diabetics are at significantly increased risk of cataract. All diabetics should have an annual measurement of visual acuity, and those with the vision of less than 6/18 in either eye should have a full eye examination, as they may have a cataract, refractive error, or glaucoma.
Recommended timing of retinal examinations in patients with type 2 diabetes[rx]
| Patient characteristics | The timing of retinal examination |
| Initial diagnosis of type 2 diabetes | Soon |
| No diabetic retinopathy | Once a year |
| Presence of symptoms such as | During the next few days |
| Loss of vision | |
| New difficulties during reading | |
| Altered color perception | |
| New, moving dark spots in the eye | |
| Presence of diabetic retinopathy | Depending on the severity of retinopathy, e.g., every 3-6 mo |
Diabetic retinopathy is detected during an eye examination that includes
- Pupil dilation –The eye care professional places drop into the eye to dilate the pupil. This allows him or her to see more of the retina and look for signs of diabetic retinopathy. After the examination, close-up vision may remain blurred for several hours.
- Ophthalmoscopy or fundus photography – Ophthalmoscopy is an examination of the retina in which the eye care professional: (1) looks through a slit lamp biomicroscope with a special magnifying lens that provides a narrow view of the retina, or (2) wearing a headset (indirect ophthalmoscope) with a bright light, looks through a special magnifying glass and gains a wide view of the retina. Hand-held ophthalmoscopy is insufficient to rule out significant and treatable diabetic retinopathy. Fundus photography generally captures considerably larger areas of the fundus and has the advantage of photo documentation for future reference, as well as availing the image to be examined by a specialist at another location and/or time.
- Fundus Fluorescein angiography (FFA) – This is an imaging technique which relies on the circulation of fluorescein dye to show staining, leakage, or non-perfusion of the retinal and choroidal vasculature.
- Optical coherence tomography (OCT) – This is an optical imaging modality based upon interference, and analogous to ultrasound. It produces cross-sectional images of the retina (B-scans) which can be used to measure the thickness of the retina and to resolve its major layers, allowing the observation of swelling.
The eye care professional will look at the retina for early signs of the disease, such as
- Leaking blood vessels,
- Retinal swelling, such as macular edema,
- Pale, fatty deposits on the retina (exudates) – signs of leaking blood vessels
- Damaged nerve tissue (neuropathy) and
- Any changes in the blood vessels.
If macular edema is suspected, FFA and sometimes OCT may be performed. Diabetic retinopathy also affects microcirculation through the body. A recent study[rx] showed assessment of conjunctival microvascular hemodynamics such as vessel diameter, red blood cell velocity, and wall shear stress can be useful for diagnosis and screening of diabetic retinopathy. Furthermore, the pattern of conjunctival microvessels was shown to be useful for rapid monitoring and diagnosis of different stages of diabetic retinopathy.[rx]
ETDRS[rx] has classified NPDR into mild, moderate, severe and very severe and PDR into early PDR and high-risk PDR. This is as follows:
-
Mild NPDR – Presence of at least one microaneurysm, definition not met for B, C, D, E, or F.
-
Moderate NPDR – Hemorrhages and/or microaneurysms more than standard photo 2A, the presence of soft exudates, venous beading, IRMA definitely present, definition not met for C, D, E, or F.
-
Severe NPDR – Hemorrhages and/or microaneurysms more than standard photo 2A in all four quadrants, or venous beading in two or more quadrants, or IRMA > standard photo 8A in at least one quadrant, definition not met for D, E, or F.
-
Very severe NPDR – Any two or more of the changes seen in severe NPDR, definition not met for E, or F.
-
Early PDR – Presence of new vessels, definition not met for F.
-
High-risk PDR – Includes any of the following characteristics – neovascularization of disc (NVD) > 1/3rd to 1/4th disc diameter, NVD < 1/3rd to 1/4th disc diameter with vitreous/ pre-retinal hemorrhage, NVE with vitreous/pre-retinal hemorrhage. High-risk characteristics (HRC) were defined by DRS, as the patient, if not treated urgently, is at high risk of severe visual loss.
-
International Clinical Diabetic Retinopathy Disease Severity scale– [rx] has developed an easily understandable scale to classify NPDR. This scale is based on findings observed upon dilated ophthalmoscopy, which includes no apparent retinopathy – no abnormalities, mild NPDR – microaneurysms only, moderate NPDR – more than just microaneurysms but less than severe NPDR and severe NPDR includes any of the following such as 20 intraretinal hemorrhages in each of four quadrants, definite venous beading in two or more quadrants, prominent IRMA in one or more quadrants and no signs of PDR.
Diabetic Macular Edema
- Macular edema or retinal thickening is an important manifestation of DR and the most common cause of moderate visual loss. The intraretinal fluid comes from leaking microaneurysms or diffuses from capillary incompetence areas. Sometimes the pockets of fluid are so large that they can be seen as cystoid macular edema (CME).
Diabetic macular edema is retinal thickening within two disc diameters of the center of the macula. DME patients were categorized into clinically significant macular edema (CSME) or non-CSME by ETDRS. CSME includes any one of the following lesions:
- Retinal thickening at or within 500 microns from the center of the macula.
- Hard exudates at or within 500 microns from the center of macula associated with thickening of the adjacent retina.
- An area or areas of retinal thickening at least one disc area in size, at least a part of which is within one disc diameter of the center of the macula.
Ancillary Investigations
- Diabetic retinopathy is essentially a clinical diagnosis. Slit lamp biomicroscopy, dilated fundus evaluation with a direct ophthalmoscope and indirect ophthalmoscope or contact/non- contact slit lamp biomicroscopic examination is essential in the diagnosis of DR. However, several ancillary investigations are required to aid the diagnosis, plan and execute the treatment and to document the lesions for research purposes. Stereoscopic fundus photographs may be required for research purposes and are especially useful for the assessment of macular edema.
Fundus Fluorescein Angiography
- Fundus fluorescein angiography (FFA) is not required for identification of lesions like NVD or NVE, as these lesions are identified clinically. FFA is used to classify and treat DME into focal and diffuse variety. It also aids in the diagnosis of CME. ETDRS has documented the angiographic risk factors for progression of NPDR to PDR.[rx] These include widespread capillary loss, capillary dilatation and fluorescein leakage as documented on FFA. It aids in differentiating IRMAs from new vessels. IRMAs do not leak on FFA while new vessels leak profusely.
Optical Coherence Tomography
- Optical coherence tomography (OCT) generates a cross-sectional image of the retina, which is comparable to histological sections. OCT is more sensitive than clinical fundus evaluation in diagnosing CSME. OCT provides us with quantitative measurement of thickness in the posterior pole area with reasonable accuracy,[rx]thus aiding in establishing the diagnosis of CSME.[rx] The repeatability and accuracy of OCT is very helpful in assessing and prognosticating the response of CSME to any treatment.[rx–rx]
- Diabetic macular edema is classified into different morphological patterns based on OCT.[rx–rx] In a study it has been shown that OCT findings correlate reasonably with FFA features.[rx]
Non-mydriatic fundus photography
- Digital non-mydriatic camera is being increasingly used for screening patients that can be subsequently reviewed by the experts to determine the need for referral to an ophthalmologist.
Treatment of Diabetic Retinopathy
Diabetic Control
As previously mentioned, good glycaemic control significantly reduces the risk of diabetic retinopathy developing and subsequently progressing. The importance of good control should be emphasized.
Laser Photocoagulation
The advent of laser photocoagulation of the retina has dramatically changed the management of diabetic retinopathy. The photocoagulation of non-proliferative diabetic retinopathy with clinically significant macular edema is called macular photocoagulation, and widespread photocoagulation for proliferative diabetic retinopathy is called pan-retinal photocoagulation.
Macular Photocoagulation
Photocoagulation for diffuse leakage around the macula may be applied in a ‘grid’ fashion to prevent leakage – grid macular photocoagulation. Diffuse or focal leakage can be identified by fundus fluorescein angiography (FFA). FFA is done with black and white retinal photography using the contrast dye, sodium fluorescein, injected into the blood.
If ‘clinically significant macular edema’ is present this may include
-
Focal leaks greater than 500μ from the center of the macula, causing retinal thickening or hard exudates
-
Focal leaks 300μ–500μ from the center of the fovea, without significant damage to the perifoveal capillary network
-
Areas of diffuse leakage on fluorescein angiography within the macular area
-
Avascular areas within the macular area.
Pan-retinal Photocoagulation (PRPC)
Photocoagulation the posterior 45°–60° of the retina, away from the vascular arcades of the macula, with graded burns – to reduce the oxygen demand of the hypoxic retina in diabetic retinopathy – converts the hypoxic zones of the retina into anoxic zones, thereby reducing the release of vaso-proliferative factors [rx]. PRPC, therefore, prevents new vessels appearing and can result in the regression of already existing new vessels on the retina or optic disc
Anti-angiogenic agents for the treatment of DR.
| Classification | Drugs | Status for DR Treatment | Clinical Benefits | Adverse Effects |
|---|---|---|---|---|
| Anti-VEGF | Ranibizumab (Lucentis) [rx,rx] | FDA approved | Greater BCVA improvement and greater reduction in CRT over laser in treating DME (DRCR.net Protocol T, RESOLVE and RESTORE trials); non-inferior to PRP in treating PDR at two years (DRCR.net Protocol S) | a. Elevation in intraocular pressure b. Vitreous hemorrhage c. Inflammation [rx] |
| Pegaptanib (Macugen) [rx] | FDA approved | Greater BCVA improvement over sham groups in treating DME (phase 2/3, multicenter, two-year trial) | a. Conjunctival hemorrhage b. Elevation in intraocular pressure |
|
| Aflibercept (EYLEA) [rx] | FDA approved | Greater BCVA improvement over laser in treating DME (VISTA, VIVID, DRCR.net Protocol T trials) and PDR (CLARITY trial) | a. Elevation in intraocular pressure b. Vitreous hemorrhage c. Inflammation [rx] |
|
| Bevacizumab (Avastin) [rx] | Off-label use | Greater reduction in CRT and better median visual acuity over laser (DRCR.net Protocol T trial) | a. Elevation in intraocular pressure b. Vitreous hemorrhage c. Inflammation |
|
| Non-specific anti-angiogenic | Squalamine (inhibits VEGF and other growth factors) [rx] | Phase 2 trial (clinicaltrials.gov ID: [rx] in progress | – | – |
| AKB-9778 (Tie2 activator) [rx] | Phase 2 trial (clinicaltrials.gov ID: [rx] in progress | Greater reduction in CRT in the combination group over ranibizumab monotherapy group (phase 2a clinical trial) | a. Diabetic retinal edema worse b. Visual acuity reduced |
|
| Nesvacumab (Anti-Ang-2) [rx] | Clinical trial (RUBY trial) in progress | Results of phase 2 RUBY trial did not provide sufficient differentiation between the combined (nesvacumab + aflibercept) and aflibercept monotherapy treatments to warrant phase 3 development | No new safety signals observed when compared with other anti-angiogenic agents | |
| RO6867461 (bispecific antibody: anti-ang-2 + anti-VEGF) | Clinical trial (BOULEVARD trial) in progress [rx] | Greater adjusted BCVA improvement and greater reduction in CRT over ranibizumab in DME patients (phase 2 BOULEVARD trial) | Well tolerated with no new safety signals observed |
Intravitreal Triamcinolone Acetonide
- Triamcinolone is a long-acting steroid preparation. When injected in the vitreous cavity, it decreases the macular edema (thickening of the retina at the macula) caused due to diabetic maculopathy and results in an increase in visual acuity. The effect of triamcinolone is transient, lasting up to three months, which necessitates repeated injections for maintaining the beneficial effect. Best results of intravitreal Triamcinolone have been found in eyes that have already undergone cataract surgery.[rx]
Intravitreal anti-VEGF
- There are good results from multiple doses of intravitreal injections of anti-VEGF drugs such as bevacizumab.[rx] A 2017 systematic review update found moderate evidence that aflibercept may have advantages in improving visual outcomes over bevacizumab and ranibizumab, after one year.[rx]Present the recommended treatment for diabetic macular edema is Modified Grid laser photocoagulation combined with multiple injections of anti-VEGF drugs.
Anti-inflammatory drugs for the treatment of DR.
| Classification | Drugs | Status for DR Treatment | Clinical Benefits | Adverse Effects |
|---|---|---|---|---|
| Intravitreal steroids | Triamcinolone [rx] | Off-label use | Greater improvements in triamcinolone + prompt laser group over laser alone in pseudophakic eyes | a. Cataract surgery b. Elevation in intraocular pressure c. Vitreous hemorrhage |
| DEX implant (Ozurdex) [rx,rx] | FDA approved | Greater BCVA improvement and greater reduction in CRT over the sham group in patients with DME (three-year trial) | a. Cataract b. Elevation in intraocular pressure c. Vitreous hemorrhage |
|
| FA insert (Iluvien, 0.2 mg) [rx] | FDA approved | Greater BCVA improvement over the sham group in patients with DME over two years | a. Cataract surgery b. Elevation in intraocular pressure c. Glaucoma |
|
| IL-6 inhibitor | EBI-031 (Rx) | Clinical trial (clinicaltrials.gov ID: [rx] in progress | – | – |
| IL-6 receptor inhibitor | Tocilizumab (Section 3.2.2) | Clinical trial (READ-4 study, clinicaltrials.gov ID: NCT02511067) in progress | – | – |
| Integrin inhibitor | Luminate (Section 3.2.2) | Phase 2b trial (PACIFIC, http://www.allegroeye.com/?s=PACIFIC) in progress | Non-inferiority to bevacizumab in mean change in BCVA and CRT for the treatment of DME (phase 2b trial, DEL MAR) | Well-tolerated with no drug toxicity or intraocular inflammation noted (phase 2b trial, DEL MAR) |
Intravitreous glucocorticoids
These are preferentially used to treat diabetic macular edema.
Anti-VEGF Drugs
- The advent of anti-VEGF therapy has revolutionized the treatment of DR. Currently, anti-VEGF drugs that have been tested in clinical trials for DR treatment include the U.S. Food and Drug Administration (FDA)-approved pegaptanib (Macugen, OSI/Eyetech, New York, NY, USA), ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA, USA), aflibercept (EYLEA; Regeneron, Tarrytown, NY, USA) and the off-label intravitreal bevacizumab
Other Anti-Angiogenic Drugs
- Currently, several anti-angiogenic drugs besides anti-VEGF agents are under clinical investigations. Squalamine demonstrated better visual recovery than control groups in patients with macular edema by inhibiting multiple angiogenic factors (VEGF, PDGF, b-FGF) [rx]. Clinical study testing the effect of squalamine in combination with ranibizumab in patients with DME is in progress (clinicaltrials.gov ID: NCT02349516).
Intravitreal Steroid
- Intravitreal corticosteroids have become increasingly important in the treatment of DME, especially in refractory DME and cases lacking a response to anti-VEGF therapy [rx]. Refractory cases of DME and nonresponders to anti-VEGF are presumed to be driven by multiple cytokines. As potent anti-inflammatory agents, corticosteroids target a broad array of mediators involved in the pathogenesis of DME including VEGF, TNF-α, chemokines, leukostasis and phosphorylation of tight-junction proteins.
Non-Steroid Anti-Inflammatory Drugs
- As one of the most important proinflammatory cytokines present in the vitreous of DR patients, IL-6 has been investigated as a promising target for anti-inflammatory therapy for DR. Antibodies against IL-6 (EBI-031) and the IL-6 receptor (tocilizumab) have been developed. Clinical trials have been carried out to test the efficacy and safety of EBI-031 (clinicaltrials.gov ID: NCT02842541) and tocilizumab (clinicaltrials.gov ID: NCT02511067) in patients with DME
Traditional Laser Treatments
- Laser photocoagulation has been the gold standard for the treatment of both DME and PDR before the advent of anti-VEGF therapy. Focal/grid macular laser therapy was shown to effectively alleviate edema of the macula and reduced the risk of moderate visual loss by 50% in the three-year Early Treatment Diabetes Retinopathy Study (ETDRS) [rx].
Cardiolipin-Targeting Peptide (MTP-131)
- Cardiolipin is a phospholipid in the inner mitochondrial membrane that might be involved in cell apoptosis [rx]. MTP-131, a selective cardiolipin-targeting peptide, showed a protective effect on visual function in a diabetic mouse model by attenuating mitochondrial oxidative stress [rx].
Alpha-Lipoic Acid
- Alpha-lipoic acid (ALA) is a mitochondria-specific antioxidant used in Alzheimer’s disease as a neuroprotective agent. It successfully prevented retinal ganglion cell loss and NFL thinning in an STZ-induced diabetic model [rx]. ALA supplementation has been shown to be associated with improved visual acuity in patients with type 1 and type 2 diabetes [rx] .
Lutein
- Lutein, a member of the carotenoid family, is a potent antioxidant accumulated in the human retina. With its anti-oxidant, anti-inflammatory and neuroprotective properties, lutein has shown a promising effect in various retinal disease models [rx,rx]. In the diabetic mouse, lutein treatment effectively prevents retinal changes [rx]. Moreover, lutein supplementation is associated with an improved visual function in patients with NPDR [rx].
ARA290
- ARA290 is small erythropoietin (EPO)-derived peptide. EPO, a mediator of erythropoiesis, has been extensively shown to have a neuroprotective role in many animal models of neurodegeneration. In diabetic rats, ARA290 showed promising efficacy in treating DR by preventing neuroglial and vascular degeneration [rx]. Currently, the effectiveness of ARA290 in the treatment of DME is under evaluation in phase 2 clinical trial.
Darapladib
- Lipoprotein-associated phospholipase A2 (Lp-PLA2) has been shown to be involved in the damage of BRB during DR. Inhibition of Lp-PLA2 in diabetic rats significantly suppressed BRB breakdown. Therefore, Lp-PLA2 may serve as a therapeutic target for the treatment of DME [rx]. Darapladib, a specific Lp-PLA2 inhibitor, demonstrated significant improvements in BCVA and macular edema in a three-month phase 2a study for the treatment of DME [rx].
- Leptin – may play a role in inciting inflammation. Leptin was found to cause upregulation of VEGF in retinal pericytes [rx], hence stimulating angiogenesis in the ischemic retina [rx], and possibly contributing to the neovascularization seen in PDR. Elevated serum and vitreous leptin were observed in patients with diabetes, and vitreous leptin was especially elevated in patients with PDR [rx]. However, cross-sectional studies could not find an association between elevated serum leptin and DR [rx, rx], though it should be noted that the sample sizes of these studies were relatively small and they may be underpowered.
- Adiponectin – has been found to induce dilation of retinal arterioles via upregulation of endothelial cell nitric oxide production, in animal studies [138]. Studies by the same group in human subjects with mild DR found that serum adiponectin was positively correlated with retinal blood flow velocity and negatively correlated with retinal arterial resistance [139]. Hence, adiponectin may have a role in countering ischemia by promoting reperfusion in the ischemic retina.
Anti-VEGF Injection Therapy
- Anti-VEGF drugs are injected into the vitreous gel to block a protein called vascular endothelial growth factor (VEGF), which can stimulate abnormal blood vessels to grow and leak fluid. Blocking VEGF can reverse abnormal blood vessel growth and decrease fluid in the retina. Available anti-VEGF drugs include Avastin (bevacizumab), Lucentis (ranibizumab), and Eylea (aflibercept). Lucentis and Eylea are approved by the U.S. Food and Drug Administration (FDA) for treating DME. Avastin was approved by the FDA to treat cancer, but is commonly used to treat eye conditions, including DME.
Focal/grid Macular Laser Surgery
- In focal/grid macular laser surgery, a few to hundreds of small laser burns are made to leaking blood vessels in areas of edema near the center of the macula. Laser burns for DME slow the leakage of fluid, reducing swelling in the retina. The procedure is usually completed in one session, but some people may need more than one treatment. Focal/grid laser is sometimes applied before anti-VEGF injections, sometimes on the same day or a few days after an anti-VEGF injection, and sometimes only when DME fails to improve adequately after six months of anti-VEGF therapy.
Potential Therapeutics for Diabetic Retinopathy
| Target | Role | Current Status | Concerns |
|---|---|---|---|
| Somatostatin | Neuroprotective, antiangiogenic. Downregulated in retinas of diabetics, associated with retinal neurodegeneration [rx]. | Recently completed multi-center phase II-III trial (EUROCONDOR) to assess the safety of topically administered somatostatin. (EudraCT Number: 2012–001200-38) Results have yet to be published. | |
| Glucagon-like peptide (GLP-1) | Neuroprotective [rx] | Intravitreal injections of exedin-4 (a GLP-1 analogue) prevent ERG abnormalities in rats with streptozotoin-induced diabetes [rx]. Topical administration of GLP-1R agonists prevents retinal neurodegeneration in mice with diabetes [rx]. | 2 large clinical trials of GLP-1 analogues in type 2 diabetics with high cardiovascular risk (LEADER and SUSTAIN-6) have shown neutral benefit [rx] or even worsening of DR compared to placebo [rx]. However, these studies were not designed to assess the progression of DR. |
| Doxycycline | Anti-inflammatory and neuroprotective [rx] | Low-dose oral doxycycline improves inner retinal function in DR compared to placebo [rx]. | Although statistical significance was achieved at multiple time points, it was a small, proof-of-concept trial. |
| Interleukin 1β (IL-1β) | Inflammatory cytokine | Systemic IL-1β inhibition has been shown to stabilize retinal neovascular changes in proliferative DR and reduce macular edema [rx]. | Open-label, small, prospective pilot study. Reduction in macular edema was not statistically significant. |
| Tumor necrosis factor α (TNF-α) | Inflammatory, induces vascular changes | Intravitreal injection of TNF-α inhibitor decreased capillary degeneration in diabetic rats [rx]. | Very small study in rats, with other primary endpoints. |
The drugs under current development for the treatment of diabetic macular edema
| Drug | Study design | Results |
| Danazol | 23 eyes: 12-wk placebo-control | Significant decreases in CRT (-86% vs -29%) and macular volume (P = 0.05) |
| Improvement in BCVA by 1 category (14% vs 47%) | ||
| Minocylcine | 5 eyes: Minocycline 100 mg BID for 6 mo | Improved BCVA and CRT compared to historic |
| controls | ||
| Loteprednol etabonate (topical) | 20 eyes: Single masked, 2-dose, randomized trial | Phase II clinical trial (KPI-121-C-004) underway |
| Dexamethasone phosphate | Iontophoresis driven into the eye EGP-437 | Positive results in 15 patients |
| PAN-90806 | 4 monotherapy arms in phase I/II trial | Maintenance therapy after single anti-VEGF injections |
| Diclofenac | 57 eyes: Intravitreal diclofenac vs bevacizumab | Diclofenac achieved better improvement in BCVA compared to bevacizumab (Δ -0.08 LogMAR vs Δ +0.04 LogMAR, P = 0.033) |
| Bevacizumab improved macular edema slightly | ||
| better | ||
| Sirolimus | Phase I trial: Dose- escalating, subconjunctival of intravitreal injections | Subconjunctival: median increase in BCVA was +4.0 letters at 45 d |
| Intravitreal: Median increase in BCVA was +4.0 letters at 90 d | ||
| Fasudil | Single intravitreal injections of fasudil with bevacizumab | At 4 wk ΔBCVA (0.84 ± 0.35 LogMAR to 0.49 ± 0.29 LogMAR; P = 0.003) and mean ΔCRT (448 ± 123 μm to 347 ± 76 μm; P = 0.001) |
| Luminate (ALG-001) | Phase IIb DME trial (targets integrin receptors) | Data expected third quarter of 2016 |
| Plasma kallekrein inhibitor (KVD001) | Phase II trials: Monotherapy for resistant DME combined with anti-VEGF | Phase I trial demonstrated safety after intravitreal injections |
| REGN910 (Ang 2 Ab) | Phase I trial | Completed. Now planning phase II trials for nAMD and DME |
Complications
Possible complications associated with diabetic retinopathy include the following:
- Vitreous hemorrhage – A newly formed blood vessel leaks into the vitreous gel that fills the eye, stopping light from reaching the retina. Symptoms include loss of vision and sensitivity to light or floaters in milder cases. This complication can resolve itself if the retina remains undamaged.
- Detached retina – Scar tissue can pull the retina away from the back of the eye. This usually causes the appearance of floating spots in the individual’s field of vision, flashes of light, and severe vision loss. A detached retina presents a significant risk of total vision loss if left untreated.
- Glaucoma – The normal flow of fluid in the eye may become blocked as new blood vessels form. The blockage causes a buildup of ocular pressure, or pressure in the eye, increasing the risk of optic nerve damage and vision loss.
References
[bg_collapse view=”button-orange” color=”#4a4949″ expand_text=”Show More” collapse_text=”Show Less” ]
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4657234/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5518557/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4398904/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2636123/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6032159/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3914226/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5488240/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5648499/
- https://www.cdc.gov/visionhealth/risk/burden.htm
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1705856/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4052230/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2828250/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4999649/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4239702/
- https://en.wikipedia.org/wiki/Diabetic_retinopathy
- https://nei.nih.gov/health/diabetic/retinopathy
- https://www.mayoclinic.org/diseases-conditions/diabetic-retinopathy/symptoms-causes/syc-20371611
- https://www.aao.org/eye-health/diseases/what-is-diabetic-retinopathy
- https://www.aoa.org/patients-and-public/eye-and-vision-problems/glossary-of-eye-and-vision-conditions/diabetic-retinopathy
- https://www.nhs.uk/conditions/diabetic-retinopathy/treatment/
- https://www.medicalnewstoday.com/articles/183417.php
[/bg_collapse]


Visitor Rating: 5 Stars