Breast cancer is the most common cancer diagnosed in women, accounting for more than 1 in 10 new cancer diagnoses each year. It is the second most common cause of death from cancer among women in the world. Anatomically, the breast has milk-producing glands in front the chest wall. They lie on the pectoralis major muscle, and there are ligaments support the breast and attach it to the chest wall. Fifteen to 20 lobes circularly arranged to form the breast. The fat that covers the lobes determines the breast size and shape. Each lobe is formed by lobules that contain the glands that are responsible for milk production in response to hormone stimulation. Breast cancer always evolves silently. Most of the patients discover their disease during their routine screening. Others may present with an accidentally discovered breast lump, change of breast shape or size, or nipple discharge. However, mastalgia is not uncommon. Physical examination, imaging especially mammography, and tissue biopsy must be done to diagnose breast cancer. The survival rate improves with early diagnosis. The tumor tends to spread lymphatically and hematologically leading to distant metastasis and poor prognosis. This explains and emphasizes the importance of breast cancer screening programs.[rx][rx][rx]
Breast cancer is cancer that develops from breast tissue.[rx] Signs of breast cancer may include a lump in the breast, a change in breast shape, dimpling of the skin, fluid coming from the nipple, a newly inverted nipple, or a red or scaly patch of skin.[rx] In those with distant spread of the disease, there may be bone pain, swollen lymph nodes, shortness of breath, or yellow skin.[rx]
WHO Classification of Breast Cancer
The 2012 World Health Organization (WHO) classification of tumors of the breast[rx] which includes benign (generally harmless) tumors and malignant (cancerous) tumors, recommends the following pathological types:
- Invasive carcinoma > Most are “not otherwise specified”
- The remainder are given subtypes:
- Pleomorphic carcinoma
- Carcinoma with osteoclast giant cells
- Carcinoma with choriocarcinoma features
- Carcinoma with melanotic features
- Invasive lobular carcinoma
Classic Solid Mixed Alveolar Tubulolobular Pleomorphic
- Tubular carcinoma
- Invasive cribriform carcinoma
- Medullary carcinoma
- Mucinous carcinoma and other tumors with abundant mucin
- Mucinous carcinoma
- Cystadenocarcinoma and columnar cell mucinous carcinoma
- Signet ring cell carcinoma
Neuroendocrine tumors
- Solid neuroendocrine carcinoma (carcinoid of the breast)
- Atypical carcinoid tumor
- Small cell/oat cell carcinoma
- Large cell neuroendocrine carcinoma
- Invasive papillary carcinoma
- Invasive micropapillary carcinoma
- Apocrine carcinoma
Metaplastic carcinomas
- Pure epithelial metaplastic carcinomas
- Squamous cell carcinoma
- Adenocarcinoma with spindle cell metaplasia
- Adenosquamous carcinoma
- Mucoepidermoid carcinoma
- Mixed epithelial/mesenchymal metaplastic carcinomas
(Other well-accepted subtypes of metaplastic mammary carcinoma thought to have clinical significance but not included in the decade-old WHO classification:
-
- Matrix-producing carcinoma
- Spindle cell carcinoma
- Carcinosarcoma
- Squamous cell carcinoma of mammary origin
- Metaplastic carcinoma with osteoclastic giant cells)
- Lipid-rich carcinoma
- Secretory carcinoma
- Oncocytic carcinoma
- Adenoid cystic carcinoma
- Acinic cell carcinoma
- Glycogen-rich clear cell carcinoma
- Sebaceous carcinoma
- Inflammatory carcinoma
- Bilateral breast carcinoma
Mesenchymal tumors (including sarcoma)
- Hemangioma
- Angiomatosis
- Hemangiopericytoma
- Pseudoangiomatous stromal hyperplasia
- Myofibroblastoma
- Fibromatosis (aggressive)
- Inflammatory myofibroblastic tumor
- Lipoma , Angiolipoma
- Granular cell tumor
- Neurofibroma
- Schwannoma
- Angiosarcoma
- Liposarcoma
- Rhabdomyosarcoma
- Osteosarcoma
- Leiomyoma
- Leiomyosarcoma
Tumors of the male breast
- Gynecomastia (benign)
- Carcinoma
- In situ
- Invasive
Malignant lymphoma
- Non-Hodgkin lymphoma
Metastatic tumors to the breast from other places in the body
Precursor lesions
- Lobular neoplasia
- lobular carcinoma in situ
- Intraductal proliferative lesions
- Usual ductal hyperplasia
- Flat epithelial hyperplasia
- Atypical ductal hyperplasia
- Ductal carcinoma in situ
- Microinvasive carcinoma
- Intraductal papillary neoplasms
- Central papilloma
- Peripheral papilloma
- Atypical papilloma
- Intraductal papillary carcinoma
- Intracystic papillary carcinoma
Benign epithelial lesions
Adenosis, including variants
- Sclerosing adenosis
- Apocrine adenosis
- Blunt duct adenosis
- Microglandular adenosis
- Adenomyoepithelial adenosis
- Radial scar / complex sclerosing lesion
Adenomas
- Tubular adenoma
- Lactating adenoma
- Apocrine adenoma
- Pleomorphic adenoma
- Ductal adenoma
Myoepithelial lesions
- Myoepitheliosis
- Adenomyoepithelial adenosis
- Adenomyoepithelioma
- Malignant myoepithelioma
Fibroepithelial tumours
- Fibroadenoma
- Phyllodes tumour
- Benign
- Borderline
- Malignant
- Periductal stromal sarcoma, low-grade
- Mammary hamartoma
Benign tumors of the nipple
- Nipple adenoma
- Syringomatous adenoma
- Paget’s disease of the nipple
Malignant tumors of the nipple
- Paget’s disease of the nipple
Types of a Breast Cancer
According to site
- Non-Invasive Breast Cancer – cells that are confined to the ducts and do not invade surrounding fatty and connective tissues of the breast. Ductal carcinoma in situ (DCIS) is the most common form of non-invasive breast cancer (90%). Lobular carcinoma in situ (LCIS) is less common and considered a marker for increased breast cancer risk.
- Invasive Breast Cancer cells that break through the duct and lobular wall and invade the surrounding fatty and connective tissues of the breast. Cancer can be invasive without being metastatic (spreading) to the lymph nodes or other organs[rx].
Frequently Occurring Breast cancer
- Lobular carcinoma in situ (LCIS, lobular neoplasia) – The term, “in situ,” refers to cancer that has not spread past the area where it initially developed. LCIS is a sharp increase in the number of cells within the milk glands (lobules) of the breast.
- Ductal carcinoma in situ (DCIS) – DCIS, the most common type of non-invasive breast cancer, is confined to the ducts of the breast. For example, ductal comedocarcinoma.
Infiltrating lobular carcinoma (ILC)
ILC is also known as invasive lobular carcinoma. ILC begins in the milk glands (lobules) of the breast but often spreads (metastasizes) to other regions of the body. ILC accounts for 10% to 15% of breast cancers.
- Infiltrating ductal carcinoma (IDC): IDC is also known as invasive ductal carcinoma. IDC begins in the milk ducts of the breast and penetrates the wall of the duct, invading the fatty tissue of the breast and possibly other regions of the body. IDC is the most common type of breast cancer, accounting for 80% of breast cancer diagnoses[rx,rx].
Less commonly occurring Breast cancer
- Medullary carcinoma – Medullary carcinoma is invasive breast cancer that forms a distinct boundary between tumor tissue and normal tissue. Only 5% of breast cancers are medullary carcinoma.
- Mutinous carcinoma – Also called colloid carcinoma, mucinous carcinoma is a rare breast cancer formed by the mucus-producing cancer cells. Women with mutinous carcinoma generally have a better prognosis than women with more common types of invasive carcinoma.
- Tubular carcinoma – Tubular carcinomas are a special type of infiltrating (invasive) breast carcinoma. Women with tubular carcinoma generally have a better prognosis than women with more common types of invasive carcinoma. Tubular carcinomas account for around 2% of breast cancer diagnoses.
Inflammatory breast cancer
- Inflammatory breast cancer is the appearance of inflamed breasts (red and warm) with dimples and/or thick ridges caused by cancer cells blocking lymph vessels or channels in the skin over the breast. Though inflammatory breast cancer is rare (accounting for only 1% of breast cancers), it is extremely fast-growing.
Paget’s disease of the nipple
- A rare form of breast cancer that begins in the milk ducts and spreads to the skin of the nipple and areola, Paget’s disease of the nipple only accounts for about 1% of breast cancers.
Phyllodes tumor
- Phyllodes tumors (also spelled “phyllodes”) are can be either benign (non-cancerous) or malignant (cancerous). Phyllodes tumors develop in the connective tissues of the breast and may be treated by surgical removal. Phyllodes tumors are very rare; less than 10 women die of this type of breast cancer each year in the United States[rx,rx,rx].
These two categories are used to describe the most common types of breast cancer, which include:
- Ductal carcinoma in situ. Ductal carcinoma in situ (DCIS) is a noninvasive condition. With DCIS, the cells that line the ducts in your breast change and look cancerous. However, DCIS cells haven’t invaded the surrounding breast tissue.
- Lobular carcinoma in situ. Lobular carcinoma in situ (LCIS) is cancer that grows in the milk-producing glands of your breast. Like DCIS, the cancer cells haven’t yet invaded the surrounding tissue.
- Invasive ductal carcinoma. Invasive ductal carcinoma (IDC) is the most common type of breast cancer. This type of breast cancer begins in your breast’s milk ducts and then invades nearby tissue in the breast. Once the breast cancer has spread to the tissue outside your milk ducts, it can begin to spread to other nearby organs and tissue.
- Invasive lobular carcinoma. Invasive lobular carcinoma (ILC) first develops in your breast’s lobules. If breast cancer is diagnosed as ILC, it has already spread to nearby tissue and organs.
Other, less common types of breast cancer include:
- Paget disease of the nipple. This type of breast cancer begins in the breasts’ ducts, but as it grows, it begins to affect the skin and areola of the nipple.
- Phyllodes tumor. This very rare type of breast cancer grows in the connective tissue of the breast.
- Angiosarcoma. This is cancer that grows on the blood vessels or lymph vessels in the breast.
The type of cancer you have determines your treatment options, as well as your prognosis (likely long-term outcome). Learn more about types of breast cancer.
Abbreviations: HER-2, human epidermal growth factor receptor 2; IBC, inflammatory breast cancer; MRI, magnetic resonance imaging; PET–CT, positron emission tomography-computed tomography.
Stage of Breast Cancer
There are 3 types of breast cancer stage groups:
-
Clinical Prognostic Stage – is used first to assign a stage for all patients based on health history, physical exam, imaging tests (if done), and biopsies. The Clinical Prognostic Stage is described by the TNM system, tumor grade, and biomarker status (ER, PR, HER2). In clinical staging, mammography or ultrasound is used to check the lymph nodes for signs of cancer.
-
Pathological Prognostic Stage – is then used for patients who have surgery as their first treatment. The Pathological Prognostic Stage is based on all clinical information, biomarker status, and laboratory test results from breast tissue and lymph nodes removed during surgery.
-
Anatomic Stage – is based on the size and the spread of cancer as described by the TNM system. The Anatomic Stage is used in parts of the world where biomarker testing is not available. It is not used in the United States.
Causes of Breast Cancer
Age
- The risk of developing breast cancer increases with age. By using the Surveillance, Epidemiology, and End Results (SEER) database, the probability of a woman in the United States developing breast cancer is a lifetime risk of 1 in 8; 1 in 202 from birth to age 39 years of age, 1 in 26 from 40-59 years, and 1 in 28 from 60-69 years[rx].
Personal history
- A personal history of breast cancer is also a significant risk factor for the development of a second ipsilateral or contralateral breast cancer. In fact, the most common cancer amongst breast cancer survivors is a metachronous contralateral breast cancer[rx]. Factors associated with an increased risk of second breast cancer include an initial diagnosis of DCIS, stage IIB, hormone receptor negative cancers, and young age[rx].
-
A personal history of invasive breast cancer, ductal carcinoma in situ (DCIS), or lobular carcinoma in situ(LCIS).
-
A personal history of benign (noncancer) breast disease.
-
A family history of breast cancer in a first-degree relative (mother, daughter, or sister).
-
Inherited changes in the BRCA1 or BRCA2 genes or in other genes that increase the risk of breast cancer.
-
Breast tissue that is dense on a mammogram.
-
Exposure of breast tissue to estrogen made by the body. This may be caused by:
-
Starting menopause at a later age.
-
Older age at first birth or never having given birth.
-
Menstruating at an early age.
-
Taking hormones such as estrogen combined with progestin for symptoms of menopause.
-
Treatment with radiation therapy to the breast/chest.
-
Drinking alcohol.
-
Obesity.
Breast pathology
- Proliferative breast disease is associated with an increased risk of breast cancer. Proliferative breast lesions without atypia, including usual ductal hyperplasia, intraductal papillomas, sclerosing adenosis and fibroadenomas confer only a small increased risk of breast cancer development, approximately 1.5-2 times that of the general population[rx].
- Atypical hyperplasia including both ductal and lobular, usually incidentally found on screening mammography, confers a substantially increased risk of breast cancer. Women with atypia have an approximately 4.3 times greater risk of developing cancer compared to the general population[rx,rx].
Genetic predisposition
- Approximately 20%-25% of breast cancer patients have a positive family history but only 5%-10% of breast cancer cases demonstrate an autosomal dominant inheritance[rx,rx]. Genetic predisposition alleles have been described in terms of clinical significance[rx]. High-risk predisposition alleles conferring a 40%-85% lifetime risk of developing breast cancer include BRCA1 and BRCA2 mutations, mutations in TP53 gene resulting in Li-Fraumeni syndrome, PTEN resulting in Cowden syndrome, STK11 causing Peutz-Jegher’s syndrome, Neurofibromatosis (NF1) and (CDH-1) E-Cadherin[rx]. Half of the breast cancer predisposition syndromes are associated with mutations in BRCA1 and BRCA2.
Early Menarche
- Early age at menarche is a risk factor among both pre- and postmenopausal women for developing breast cancer. Delay in menarche by two years is associated with a corresponding risk reduction of 10%[rx].
- Within the European Prospective Investigation into Cancer and Nutrition cohort, women who had early menarche (≤ 13 years) demonstrated a nearly twofold increase in the risk of hormone receptor positive tumors[rx].
Parity and age at first full-term pregnancy
- Nulliparous women are at an increased risk for the development of breast cancer compared to parous women. Young age at first birth has an overall protective effect, whereas a relatively advanced age at first birth confers a relative risk of breast cancer greater than that of a nulliparous woman.
- Compared to nulliparous women the cumulative incidence of breast cancer in women experiencing their first birth at age 20, 25, and 35 years was 20% lower, 10% lower and 5% higher, respectively[rx].
Breast feeding
- Evidence suggests that breast feeding has a protective effect against the development of breast cancer. Breast feeding may delay the return of regular ovulatory cycles and decrease endogenous sex hormone levels. It has been estimated that there is a 4.3% reduction for every one-year of breast feeding[rx].
Testosterone
- High endogenous sex hormone levels increase the risk of breast cancer in both premenopausal and postmenopausal women. High levels of circulating testosterone in postmenopausal women have been linked to increased risk of developing breast cancer [rx].
Alcohol Consumption
- Alcohol consumption has been associated with increased breast cancer risk that is statistically significant at levels as low. Binge drinking, but not the frequency of drinking, was associated with breast cancer risk after controlling for cumulative alcohol intake. Alcohol intake both earlier and later in adult life was independently associated with risk[rx].
Physical activity
- Consistent physical activity has been shown to reduce the risk of breast cancer in a dose-dependent manner, with modest activity conferring a 2% decrease in risk and vigorous activity a 5% decrease in risk[rx].
Obesity
- Obesity, specifically in postmenopausal women, has also been shown to increase a woman’s risk of breast cancer. In the EPIC multicenter prospective cohort study, postmenopausal women who did not use HRT had elevated breast cancer risk with increasing weight, body mass index (BMI) and hip circumference[rx].
- In this cohort, the multivariate relative risk was 1.28 for overweight women (BMI 25.0-29.9) and obese women (BMI > 30.0) compared to women in the normal weight range. Lean women on HRT are incongruously at an increased risk of breast cancer (RR = 2.04) compared to their overweight (1.93) and obese (1.39) counterparts[rx].
Radiation
- Radiation exposure from various sources including medical treatment and nuclear explosion increases the risk of breast cancer. Radiation to the chest wall for treatment of childhood cancer increases the risk of breast cancer linearly with chest radiation dose[rx].
- Personal history of breast cancer – A history of cancer in one breast increases the likelihood of a second primary cancer in the contralateral breast.
- Histologic risk factors – Histologic abnormalities diagnosed by breast biopsy constitute an important category of breast cancer risk factors. These abnormalities include lobular carcinoma in situ (LCIS) and proliferative changes with atypia.
- The family history of breast cancer and genetic risk factors – First-degree relatives of patients with breast cancer have a 2-fold to 3-fold excess risk for development of the disease. Five percent to 10% of all breast cancer cases are due to genetic factors, but they may account for 25% of cases in women younger than 30 years. BRCA1 and BRCA2 are the 2 most important genes responsible for increased breast cancer susceptibility.
- Reproductive risk factors – Reproductive milestones that increase a woman’s lifetime estrogen exposure are thought to increase her breast cancer risk. These include the onset of menarche before 12 years of age, first live childbirth after age 30 years, nulliparity, and menopause after age 55 years.
- Exogenous hormone use – Therapeutic or supplemental estrogen and progesterone are taken for various conditions, with the two most common scenarios being contraception in premenopausal women and hormone replacement therapy in postmenopausal women.
Risk factors for breast cancer include the following:
-
A personal history of invasive breast cancer, ductal carcinoma in situ (DCIS), or lobular carcinoma in situ(LCIS).
-
A personal history of benign (noncancer) breast disease.
-
A family history of breast cancer in a first-degree relative (mother, daughter, or sister).
-
Inherited changes in the BRCA1 or BRCA2 genes or in other genes that increase the risk of breast cancer.
-
Breast tissue that is dense on a mammogram.
-
Exposure of breast tissue to estrogen made by the body. This may be caused by:
-
Starting menopause at a later age.
-
Older age at first birth or never having given birth.
-
Menstruating at an early age.
-
Taking hormones such as estrogen combined with progestin for symptoms of menopause.
-
Treatment with radiation therapy to the breast/chest.
-
Drinking alcohol.
-
Obesity.
The use of certain medicines and other factors decrease the risk of breast cancer.
Anything that decreases your chance of getting a disease is called a protective factor.
Protective factors for breast cancer include the following:
- Taking any of the following:
- Estrogen-only hormone therapy after a hysterectomy.
- Selective estrogen receptor modulators (SERMs).
- Aromatase inhibitors.
- Less exposure of breast tissue to estrogen made by the body. This can be a result of:
- Early pregnancy.
- Breastfeeding.
-
Getting enough exercise.
- Having any of the following procedures:
- Mastectomy to reduce the risk of cancer.
- Oophorectomy to reduce the risk of cancer.
- Ovarian ablation.
Increasing age is the most important risk factor for most cancers. Other risk factors for breast cancer include the following:
- Major inheritance susceptibility.[rx,rx]
- Germline mutation of the BRCA1 and BRCA2 genes and other breast cancer susceptibility genes.[rx]
-
Alcohol intake.
-
Breast tissue density (mammographic).[rx]
- Estrogen (endogenous).[rx–rx]
- Menstrual history (early menarche/late menopause).[rx,rx]
- Nulliparity.
- Older age at first birth.
- Hormone therapy history.
- Combination estrogen plus progestin hormone replacement therapy.
-
Obesity (postmenopausal).[rx]
-
Personal history of breast cancer.[rx]
- Radiation exposure to breast/chest.
- Age-specific risk estimates are available to help counsel and design screening strategies for women with a family history of breast cancer.[rx,rx]
Symptoms of Breast Cancer
Breast cancer is usually not painful in the early stages. But some things may be signs of breast cancer – or a non-cancerous lump. It is important to see your doctor very soon if you notice any of the following changes:
-
One breast changes size or shape
-
You can feel a lump in a breast or armpit
-
There is a sunken dip (dimple) on the nipple or elsewhere on the breast
-
Red or scaly skin on a breast that doesn’t go away
-
A clear or bloody fluid comes out of a nipple
-
A lump or thickening in or near the breast or in the underarm area.
-
A change in the size or shape of the breast.
-
A dimple or puckering in the skin of the breast.
-
A nipple turned inward into the breast.
-
Fluid, other than breast milk, from the nipple, especially if it’s bloody.
-
Scaly, red, or swollen skin on the breast, nipple, or areola (the dark area of skin around the nipple).
-
Dimples in the breast that look like the skin of an orange, called peau d’orange.
- a breast lump or tissue thickening that feels different than surrounding tissue and has developed recently
- breast pain
- red, pitted skin over your entire breast
- swelling in all or part of your breast
- a nipple discharge other than breast milk
- bloody discharge from your nipple
- peeling, scaling, or flaking of skin on your nipple or breast
- a sudden, unexplained change in the shape or size of your breast
- inverted nipple
- changes to the appearance of the skin on your breasts
- a lump or swelling under your arm
- A breast lump or thickening that feels different from the surrounding tissue
- Change in the size, shape or appearance of a breast
- Changes to the skin over the breast, such as dimpling
- A newly inverted nipple
- Peeling, scaling, crusting or flaking of the pigmented area of skin surrounding the nipple (areola) or breast skin
- Redness or pitting of the skin over your breast, like the skin of an orange
Diagnosis of Breast Cancer
Your doctor is the first person to go to if you think you might have breast cancer. After discussing previous and/or current medical conditions (your medical history) with the doctor, he or she will perform a physical exam. This may include the following:
-
Feeling (palpating) the breast and armpits
-
Breast x-ray (mammography)
-
Ultrasound (sonography)
-
Magnetic resonance imaging (MRI)
-
Biopsy (taking a tissue sample for lab analysis)
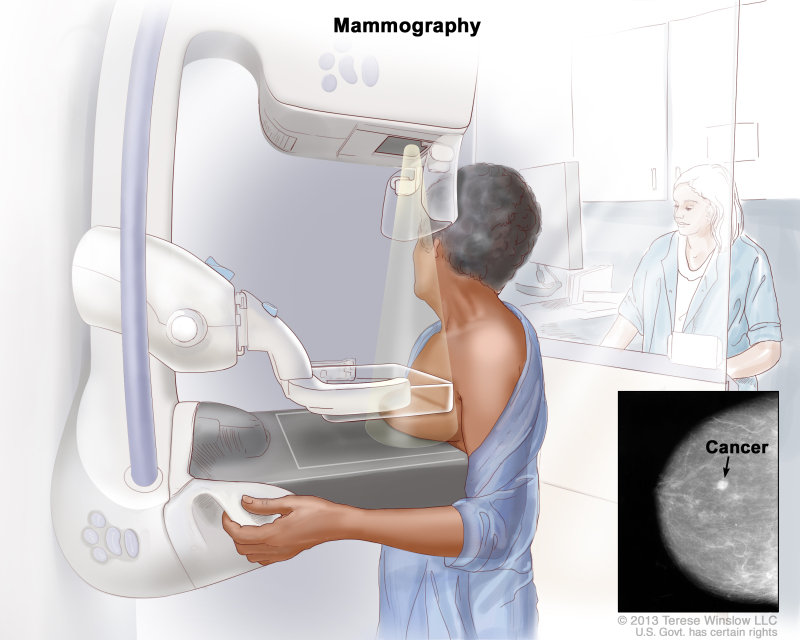
Mammography
- A mammogram is an X-ray picture of the breast.[rx] Digital mammography has replaced conventional (film screen) mammography in some breast screening services.[rx] Potential advantages of DM include the use of computer-aided detection, algorithm-based computer programs that alert the radiologist to possible abnormalities on the mammogram and allowing centralized film reading.[rx]
- Mammography frequent use, however, warrants diligent analysis of potential radiation risk. Moreover, false-positive calls lead to additional imaging or histopathological assessment, mainly percutaneous breast biopsy.[rx]
Magnetic resonance imaging (MRI)
- MRI is a powerful imaging tool that produces high-resolution images without requiring the application of harmful radiation. This technique is similar to nuclear magnetic resonance where a proton density image of the tissue is studied to generate an MRI image.
- MRI of the breast is not routinely used in breast diagnosis.[rx],[rx] National Comprehensive Cancer Network considers breast MRI as a useful adjunct to diagnostic mammography, if needed, in some specific situations due to poor selectivity and its dependence contrast media.[rx],[rd] In spite of its low selectivity, MRI high sensitivity enables breast cancer early diagnosis.[rx] Van Goethem et al also reported the high sensitivity of MRI in the detection of IDC and the staging of breast cancer.[rx]
Breast self- and clinical breast examination
- The utility of the breast self-examination (BSE) is controversial as the benefit in terms of decreased mortality has not been demonstrated[rx]. Most clinicians encourage women to perform monthly BSE to become familiar with their normal anatomy and empower them with regards to their own healthcare[rx].
- The 2013 NCCN guidelines recommend annual clinical breast examination (CBE) for women of average risk > 40 years of age as well as BSE to develop and exhibit breast self-awareness[rx].
Mammography
- One of the most important advances in the treatment of breast cancer is early detection of non-palpable masses. In the 1960s, the first randomized control trials comparing periodic mammography screening clinical examination demonstrated a decreased mortality by approximately one third in the experimental group.
- However, there is still controversy regarding mortality from breast cancer in the subset of women aged 40-49 years[rx–rx]. Contemporary randomized control trials have demonstrated the benefits of screening mammography in women aged 40 to 70 years[rx–rx].
Ultrasound
-
A procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram. The picture can be printed to be looked at later.
- There are several studies supporting the use of adjunctive screening ultrasound in high-risk patients with dense breast tissue, which imparts a substantial but accepted number of false positives[rx]. No randomized controlled trials have been conducted to evaluate the impact of screening ultrasonography on breast cancer mortality rates.
- Whole breast ultrasound may allow the clinician to screen for breast cancers not detected by traditional mammography, especially in dense breasts where mammographic sensitivity is lower[rx]. Single center studies have shown that the incremental detection of breast cancer by ultrasound
Molecular breast imaging (MBI)
- MBI uses a radioactive tracer that lights up cancer tissues of the breast, visualized by a nuclear medicine scanner.[rx] This technique is also called Miraluma test, sestamibi test, scintimammography, or specific gamma imaging. MBI depends mainly on Tc-99m sestamibi, which is approved for breast cancer imaging.[rx] MBI has comparable sensitivity to MRI and rather a higher specificity that can detect small breast lesions.[rx]
Breast Biopsy
- The only definitive method for diagnosing breast cancer is with a breast biopsy. There are several different types of breast biopsies.[rx] To increase diagnostic accuracy and eliminate as many false negative results as possible, clinical breast examination, breast imaging, and biopsy are performed simultaneously (triple test).[rx]
Needle Biopsy
- Two types of needle biopsies are used to diagnose breast cancer: fine needle aspiration cytology (FNAC) and core needle biopsy (CNB).[rx],[rx] FNAC is the least invasive method of breast biopsy.[rx] With FNAC, a thin, hollow needle is inserted into the breast to withdraw cells from the suspicious lesion. The cells are then submitted to a laboratory for analysis. FNAC can be conducted rapidly and easily, and quick smears can be used to assess the adequacy of the tissue sample.[rx]
- CNB uses a larger needle than FNAC, and instead of cells, CNB removes a small cylinder of tissue (a core) about the size of a grain of rice.[rx] About three to five cores are usually removed, although more may be taken.[rx] The core tissue samples are then analyzed by a pathologist for malignant cells.[rx]
Immunohistochemistry (IHC)
- IHC is a technique that uses antibodies as a tool to detect protein expression.[rx] Monoclonal or polyclonal antibodies complementary to the antigen of interest are labeled with a marker (either visible by light microscopy or fluorescence), allowing detection of the antibodies bound to regions of protein expression in a tissue sample.[rx] Diagnostic IHC is widely used, for example, to detect tissue markers associated with specific cancer.[rx]
FISH test
- FISH is a technique used to identify the presence of specific chromosomes or chromosomal regions through hybridization (attachment) of fluorescently labeled DNA probes to denatured chromosomal DNA.[rx] Examination under fluorescent lighting detects the presence of the hybridized fluorescent signal (and hence the presence of the chromosome material).[rx]
Serum tumor biomarkers
- Breast biomarkers are CA 15-3, carcinoembryonic antigen (CEA), and CA 27-29.[rx] All have low sensitivity and specificity and thus are not helpful in the early detection of breast cancer.[rx] The American Society of Clinical Oncology recommends the use of CEA, CA 15-3, and CA 27–29 only in metastatic settings.[rx]
Proteins
- Mammaglobin is a protein found in mammary tissue and can be detected in serum.[rx] Galvis-Jimenez et al managed to detect mammaglobins in 51 breast cancer patients using ELISA.[rx] Moreover, S100A11, a Ca++ binding protein, was suggested by Liu et al as an effective tool to help in the detection of early stage breast cancer because of its high expression in early stages.[rx]
Cancer cells
- Circulating endothelial cells (CECs), as well as bone marrow-derived endothelial precursor cells (EPCs), play an important role in neovascularization and tumor growth.[rx] CEC and EPC are good candidates for screening breast cancer and even better candidates for monitoring the antiangiogenic treatment.[rx]
- Other cells that may be used are cancer stem-like cells (CSCs). Chang et al reported that leptin, an obesity-associated adipokine, regulates a transcriptional pathway to silence a genetic program of epithelial homeostasis in breast cancer CSC that promotes malignant progression.[rx]
DNA and RNA
- Apoptosis and necrosis of the cancer tissue lead to elevated free DNA/RNA in the blood of the patients by 50-folds.[rx] Epigenetic analysis of abnormal DNA methylation has been promising in the detection of breast cancer. Hypermethylation of a gene is associated with the loss of expression and can inactivate tumor suppressor genes or other cancer genes.[rx]
- Recently, Heyn et al in a cohort study proved that hypermethylation of DOK7 (Docking Protein 7) occurs years before tumor diagnosis and thus acts as a powerful epigenetic blood-based biomarker as well as provides insights into breast cancer pathogenesis.[rx]
Autoantibody
- Antibodies may reflect the immune response to the earliest cancer cells or alternatively a robust antitumor defense associated with reduced risk of developing cancer.[rx] Autoantibodies directed against tumor-associated antigens (TAAs) have been shown to be relevant tumor markers.[rx]
- The combination of serologic biomarkers of TAAs with autoantibodies may improve the diagnostic accuracy of breast cancer.[rx] Liu et al suggest that autoantibodies against p90/CIP2A may be a useful serum biomarker for early-stage breast cancer screening and diagnosis.[rx]
Genomic and proteomics
- Genomic studies have produced a number of useful tissue-based gene signatures that can predict prognosis.[rx] Two of these are already in clinical use for a subset of breast cancer patients: the Oncotype DX test54 and the Mammaprint assay.[rx]
- Blood-based proteomics has identified several potential biomarkers, including HSP27, transcriptional regulator 14-3-3 σ, derivatives of the complement component C3a, and a fragment of fibrinogen-α.[rx]
- Furthermore, numerous proteomic studies of breast cancer have been accomplished aiming to aid the development of personalized therapies, increase understanding of post-treatment relapse, and help improve prediction of patient prognosis.[rx]
Tests that examine the breasts are used to detect (find) and diagnose breast cancer.
Check with your doctor if you notice any changes in your breasts. The following tests and procedures may be used:
-
Physical exam and history – An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
-
Clinical breast exam (CBE) – An exam of the breast by a doctor or other health professional. The doctor will carefully feel the breasts and under the arms for lumps or anything else that seems unusual.
-
Blood chemistry studies: A procedure in which a blood sample is checked to measure the amounts of certain substances released into the blood by organs and tissues in the body. An unusual (higher or lower than normal) amount of a substance can be a sign of disease.
-
Biopsy – The removal of cells or tissues so they can be viewed under a microscope by a pathologist to check for signs of cancer. If a lump in the breast is found, a biopsy may be done.There are four types of biopsy used to check for breast cancer:
-
Excisional biopsy – The removal of an entire lump of tissue.
-
Incisional biopsy – The removal of part of a lump or a sample of tissue
-
Core biopsy – The removal of tissue using a wide needle
-
Fine-needle aspiration (FNA) biopsy – The removal of tissue or fluid, using a thin needle.
If cancer is found, tests are done to study the cancer cells.
Decisions about the best treatment are based on the results of these tests. The tests give information about:
-
how quickly cancer may grow.
-
how likely it is that cancer will spread through the body.
-
how well certain treatments might work.
-
how likely the cancer is to recur (come back).
Tests include the following
-
Estrogen and progesterone receptor test – A test to measure the amount of estrogen and progesterone (hormones) receptors in cancer tissue. If there are more estrogen and progesterone receptors than normal, the cancer is called estrogen and/or progesterone receptor positive. This type of breast cancer may grow more quickly. The test results show whether treatment to block estrogen and progesterone may stop cancer from growing.
-
Human epidermal growth factor type 2 receptor (HER2/neu) test – A laboratory test to measure how many HER2/neu genes there are and how much HER2/neu protein is made in a sample of tissue. If there are more HER2/neu genes or higher levels of HER2/neu protein than normal, the cancer is called HER2/neu positive. This type of breast cancer may grow more quickly and is more likely to spread to other parts of the body. Cancer may be treated with drugs that target the HER2/neu protein, such as trastuzumab and pertuzumab.
-
Multigene tests – Tests in which samples of tissue are studied to look at the activity of many genes at the same time. These tests may help predict whether cancer will spread to other parts of the body or recur (come back).There are many types of multigene tests. The following multigene tests have been studied in clinical trials:
-
Oncotype DX – This test helps predict whether stage I or stage II breast cancer that is estrogen receptor positive and node-negative will spread to other parts of the body. If the risk that cancer will spread is high, chemotherapy may be given to lower the risk.
-
MammaPrint: This test helps predict whether stage I or stage II breast cancer that is node-negative will spread to other parts of the body. If the risk that cancer will spread is high, chemotherapy may be given to lower the risk.
Based on these tests, breast cancer is described as one of the following types:
-
Hormone receptor positive (estrogen and/or progesterone receptor positive) or hormone receptor negative (estrogen and/or progesterone receptor negative).
-
HER2/neu positive or HER2/neu negative.
-
Triple negative (estrogen receptor, progesterone receptor, and HER2/neu negative).
This information helps the doctor decide which treatments will work best for your cancer.
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis (chance of recovery) and treatment options depend on the following:
-
The stage of cancer (the size of the tumor and whether it is in the breast only or has spread to lymph nodes or other places in the body).
-
The type of breast cancer.
-
Estrogen receptor and progesterone receptor levels in the tumor tissue.
-
Human epidermal growth factor type 2 receptor (HER2/neu) levels in the tumor tissue.
-
Whether the tumor tissue is triple-negative (cells that do not have estrogen receptors, progesterone receptors, or high levels of HER2/neu).
-
How fast the tumor is growing.
-
How likely the tumor is to recur (come back).
-
A woman’s age, general health, and menopausal status (whether a woman is still having menstrual periods).
-
Whether cancer has just been diagnosed or has recurred (come back).
The following tests and procedures also may be used in the staging process:
-
Sentinel lymph node biopsy – The removal of the sentinel lymph node during surgery. The sentinel lymph node is the first lymph node in a group of lymph nodes to receive lymphatic drainage from the primary tumor. It is the first lymph node the cancer is likely to spread to from the primary tumor. A radioactive substance and/or blue dye is injected near the tumor. The substance or dye flows through the lymph ducts to the lymph nodes. The first lymph node to receive the substance or dye is removed. A pathologist views the tissue under a microscope to look for cancer cells. If cancer cells are not found, it may not be necessary to remove more lymph nodes. Sometimes, a sentinel lymph node is found in more than one group of nodes.
-
Chest x-ray – An x-ray of the organs and bones inside the chest. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
-
CT scan (CAT scan) – A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
-
Bone scan – A procedure to check if there are rapidly dividing cells, such as cancer cells, in the bone. A very small amount of radioactive material is injected into a vein and travels through the bloodstream. The radioactive material collects in the bones with cancer and is detected by a scanner.
-
PET scan (positron emission tomography scan) – A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
There are three ways that cancer spreads in the body.
Cancer can spread through tissue, the lymph system, and the blood:
-
Tissue – Cancer spreads from where it began by growing into nearby areas.
-
Lymph system – Cancer spreads from where it began by getting into the lymph system. The cancer travels through the lymph vessels to other parts of the body.
-
Blood – Cancer spreads from where it began by getting into the blood. Cancer travels through the blood vessels to other parts of the body.
Cancer may spread from where it began to other parts of the body.
When cancer spreads to another part of the body, it is called metastasis. Cancer cells break away from where they began (the primary tumor) and travel through the lymph system or blood.
-
Lymph system – Cancer gets into the lymph system, travels through the lymph vessels, and forms a tumor(metastatic tumor) in another part of the body.
-
Blood – Cancer gets into the blood, travels through the blood vessels, and forms a tumor (metastatic tumor) in another part of the body.
-
The metastatic tumor is the same type of cancer as the primary tumor. For example, if breast cancer spreads to the bone, the cancer cells in the bone are actually breast cancer cells. The disease is metastatic breast cancer, not bone cancer.
metastasis: how cancer spreads
Many cancer deaths are caused when cancer moves from the original tumor and spreads to other tissues and organs. This is called metastatic cancer. This animation shows how cancer cells travel from the place in the body where they first formed to other parts of the body. To plan the best treatment and understand your prognosis, it is important to know the breast cancer stage.
Treatment
Drugs Approved to Treat Breast Cancer
- Abemaciclib
- Abraxane (Paclitaxel Albumin-stabilized Nanoparticle Formulation)
- Ado-Trastuzumab Emtansine
- Afinitor (Everolimus)
- Anastrozole
- Aredia (Pamidronate Disodium)
- Arimidex (Anastrozole)
- Aromasin (Exemestane)
- Capecitabine
- Cyclophosphamide
- Docetaxel
- Doxorubicin Hydrochloride
- Ellence (Epirubicin Hydrochloride)
- Epirubicin Hydrochloride
- Eribulin Mesylate
- Everolimus
- Exemestane
- 5-FU (Fluorouracil Injection)
- Fareston (Toremifene)
- Faslodex (Fulvestrant)
- Femara (Letrozole)
- Fluorouracil Injection
- Fulvestrant
- Gemcitabine Hydrochloride
- Gemzar (Gemcitabine Hydrochloride)
- Goserelin Acetate
- Halaven (Eribulin Mesylate)
- Herceptin (Trastuzumab)
- Ibrance (Palbociclib)
- Ixabepilone
- Ixempra (Ixabepilone)
- Kadcyla (Ado-Trastuzumab Emtansine)
- Kisqali (Ribociclib)
- Lapatinib Ditosylate
- Letrozole
- Lynparza (Olaparib)
- Megestrol Acetate
- Methotrexate
- Neratinib Maleate
- Nerlynx (Neratinib Maleate)
- Olaparib
- Paclitaxel
- Paclitaxel Albumin-stabilized Nanoparticle Formulation
- Palbociclib
- Pamidronate Disodium
- Perjeta (Pertuzumab)
- Pertuzumab
- Ribociclib
- Tamoxifen Citrate
- Taxol (Paclitaxel)
- Taxotere (Docetaxel)
- Thiotepa
- Toremifene
- Trastuzumab
- Trexall (Methotrexate)
- Tykerb (Lapatinib Ditosylate)
- Verzenio (Abemaciclib)
- Vinblastine Sulfate
- Xeloda (Capecitabine)
- Zoladex (Goserelin Acetate)
Lumpectomy with surgical axillary staging
- Negative axillary nodes – Radiation therapy to the whole breast with or without boost (by photons, brachytherapy, or electron beam) to tumor bed or consideration of partial breast irradiation (PBI) in selected patients. Radiation therapy should follow chemotherapy when chemotherapy is indicated.
- One-three positive axillary nodes – Radiation therapy to the whole breast with or without boost (by photons, brachytherapy, or electron beam) to tumor bed following chemotherapy when chemotherapy is indicated. Strongly consider radiation therapy to the infraclavicular region and supraclavicular area. Strongly consider radiation therapy to internal mammary nodes. Radiation therapy should follow chemotherapy when chemotherapy is indicated.
- > Four positive axillary nodes: Radiation therapy to the whole breast with or without boost (by photons, brachytherapy, or electron beam) to the tumor bed, infraclavicular region, and supraclavicular area. Strongly consider radiation therapy to internal mammary nodes. Radiation therapy should follow chemotherapy when chemotherapy is indicated.
Total mastectomy with surgical axillary staging ± reconstruction
- No radiation therapy: Negative axillary nodes and tumor ≤ 5 cm and margins ≥ 1 mm.
- Consider postchemotherapy radiation therapy to chest wall: Negative axillary nodes and tumor ≤ 5 cm and close margins (< 1 mm).
- Strongly consider radiation therapy to internal mammary nodes: Negative axillary nodes and tumor > 5 cm ormargins positive: Consider radiation therapy to chest wall ± infraclavicular.
- One-three positive axillary nodes: Strongly consider postchemotherapy radiation therapy to chest wall + infraclavicular and supraclavicular areas; if radiation therapy is given, strongly consider internal mammary node radiation therapy.
- ≥ Four positive axillary nodes: Postchemotherapy radiation therapy to chest wall + infraclavicular and supraclavicular areas. Strongly consider radiation therapy to internal mammary nodes.
Systemic Pharmacotherapy
Although surgery and radiation address local disease in the breast and regional disease in the lymph node beds, systemic therapy addresses microscopic disease elsewhere that can become metastases. When patients die from breast cancer, the cause is widespread metastatic disease. It is pharmacotherapy that ultimately improves breast cancer survival rates since this is the only treatment directed at the systemic disease. Pharmacotherapies for breast cancer consist of endocrine (hormonal) therapy, cytotoxic chemotherapy, and biological targeted (antibody) therapies.
-
Endocrine therapy – requires relatively few specialized resources, but it requires knowledge of hormone receptor status to identify the patients most likely to benefit. For ER-positive cancers, tamoxifen and aromatase inhibitors are oral drugs taken daily for five years or more that can be dispensed from pharmacies without special infrastructure and are considered very safe. Endocrine therapy could be given to all breast cancer patients, but it would be a waste of resources since it is only effective against ER-positive cancers. IHC methods involve special tissue-staining techniques with labeling antibodies, which requires pathology laboratory infrastructure; quality control is quite important to testing accuracy. Alternative simplified techniques for ER testing are of significant interest but remain experimental.
-
Systemic cytotoxic chemotherapy – is effective in most biologic subtypes of breast cancer. It is particularly important in the management of ER-negative cancers but is resource intensive. Chemotherapy has significant side effects that must be managed effectively. Correct drug selection is based on the extent or stage of cancer and on tumor markers that can predict likely drug sensitivity. Proper drug dosing is important and must be individualized to the patient’s body mass index; the dosage should be sufficiently high to provide optimal effects on cancer but as low as possible to minimize adverse events. Proper management of these agents is critical; they must be handled under sterile conditions, they must be properly and safely administered, and health care workers should not be directly exposed to these agents.
-
Biological targeted therapies – use monoclonal antibodies to control disease. HER2 neu-targeted therapy with trastuzumab is very effective in tumors that overexpress the HER2/neu oncogene, but cost largely prevents the use of this treatment in LMICs (Eniu and others 2008); a standard one-year course of treatment in the United States is approximately US$100,000. It remains unclear whether generic forms of trastuzumab will be available in the future.
Estrogen-only hormone therapy after hysterectomy
- Hormone therapy with estrogen only may be given to women who have had a hysterectomy. In these women, estrogen-only therapy after menopause may decrease the risk of breast cancer. There is an increased risk of stroke and heart and blood vessel disease in postmenopausal women who take estrogen after a hysterectomy.
Selective estrogen receptor modulators
- Tamoxifen and raloxifene [Tamoxifen 20 mg tablet {General Pharm Tamofen 20} – 20mg dailyin a single dosage or in 2 divided dosage. The maximum dosage are 40 mg in divided dosage. or raloxifene is altenate option]. belong to the family of drugs called selective estrogen receptor modulators (SERMs). SERMs act like estrogen on some tissues in the body but block the effect of estrogen on other tissues.
- Treatment with tamoxifen lowers the risk of estrogen receptor-positive (ER-positive) breast cancer and ductal carcinoma in situ in premenopausal and postmenopausal women at high risk. Treatment with raloxifene also lowers the risk of breast cancer in postmenopausal women. With either drug, the reduced risk lasts for several years or longer after treatment is stopped. Lower rates of broken bones have been noted in patients taking raloxifene.
Aromatase inhibitors and inactivators
Aromatase inhibitors (anastrozole, letrozole)[Tab: Letrozole 2.5 mg { Letrol /Lerozol 2.5 mg Square pharma} (0+0+1)]and inactivators (exemestane) lower the risk of recurrence and of new breast cancers in women who have a history of breast cancer. Aromatase inhibitors also decrease the risk of breast cancer in women with the following conditions:
-
Postmenopausal women with a personal history of breast cancer.
-
Women with no personal history of breast cancer who are 60 years and older, have a history of ductal carcinoma in situ with mastectomy or have a high risk of breast cancer based on the Gail model tool (a tool used to estimate the risk of breast cancer).
Surgery
Most patients with breast cancer have surgery to remove cancer.
Sentinel lymph node biopsy – is the removal of the sentinel lymph node during surgery. The sentinel lymph node is the first lymph node in a group of lymph nodes to receive lymphatic drainage from the primary tumor. It is the first lymph node the cancer is likely to spread to from the primary tumor. A radioactive substance and/or blue dye is injected near the tumor. The substance or dye flows through the lymph ducts to the lymph nodes. The first lymph node to receive the substance or dye is removed. A pathologist views the tissue under a microscope to look for cancer cells. If cancer cells are not found, it may not be necessary to remove more lymph nodes. Sometimes, a sentinel lymph node is found in more than one group of nodes. After the sentinel lymph node biopsy, the surgeon removes the tumor using breast-conserving surgery or mastectomy. If cancer cells were found, more lymph nodes will be removed through a separate incision. This is called a lymph node dissection.
Types of surgery include the following:
Breast-conserving surgery – is an operation to remove cancer and some normal tissue around it, but not the breast itself. Part of the chest wall lining may also be removed if the cancer is near it. This type of surgery may also be called lumpectomy, partial mastectomy, segmental mastectomy, quadrantectomy, or breast-sparing surgery.
-
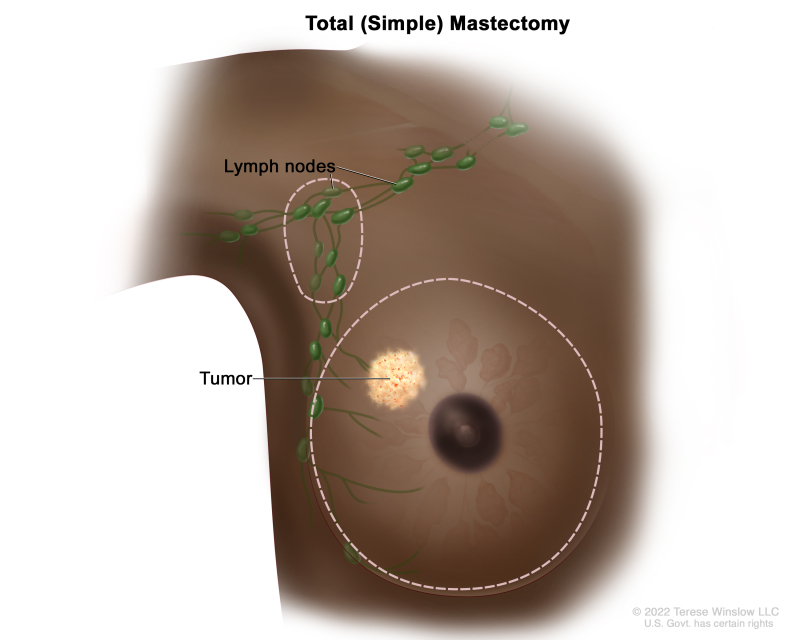
Total mastectomy – Surgery to remove the whole breast that has cancer. This procedure is also called a simple mastectomy. Some of the lymph nodes under the arm may be removed and checked for cancer. This may be done at the same time as the breast surgery or after. This is done through a separate incision.
Chemotherapy – may be given before surgery to remove the tumor. When given before surgery, chemotherapy will shrink the tumor and reduce the amount of tissue that needs to be removed during surgery. Treatment is given before surgery is called preoperative therapy or neoadjuvant therapy.
After the doctor removes all cancer that can be seen at the time of the surgery, some patients may be given radiation therapy, chemotherapy, targeted therapy, or hormone therapy after surgery, to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that cancer will come back, is called postoperative therapy or adjuvant therapy.
FDA website for more information on breast implants.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy:
-
External radiation therapy uses a machine outside the body to send radiation toward cancer.
-
Internal radiation therapy uses a radioactive substance sealed in needles, seeds, wires, or catheters that are placed directly into or near cancer.
-
The way the radiation therapy is given depends on the type and stage of the cancer being treated. External radiation therapy is used to treat breast cancer. Internal radiation therapy with strontium-89 (a radionuclide) is used to relieve bone pain caused by breast cancer that has spread to the bones. Strontium-89 is injected into a vein and travels to the surface of the bones. Radiation is released and kills cancer cells in the bones.
Chemotherapy
- Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy).
- When chemotherapy is placed directly into the cerebrospinal fluid, an organ, or a body cavity such as the abdomen, the drugs mainly affect cancer cells in those areas (regional chemotherapy).The way the chemotherapy is given depends on the type and stage of the cancer being treated. Systemic chemotherapy is used in the treatment of breast cancer.
See Drugs Approved for Breast Cancer for more information.
Hormone therapy
- Hormone therapy is a cancer treatment that removes hormones or blocks their action and stops cancer cells from growing. Hormones are substances made by glands in the body and circulated in the bloodstream. Some hormones can cause certain cancers to grow. If tests show that the cancer cells have places where hormones can attach (receptors), drugs, surgery, or radiation therapy is used to reduce the production of hormones or block them from working. The hormone estrogen, which makes some breast cancers grow, is made mainly by the ovaries. Treatment to stop the ovaries from making estrogen is called ovarian ablation.
- Hormone therapy with tamoxifen is often given to patients with early localized breast cancer that can be removed by surgery and those with metastatic breast cancer (cancer that has spread to other parts of the body). Hormone therapy with tamoxifen or estrogens can act on cells all over the body and may increase the chance of developing endometrial cancer. Women taking tamoxifen should have a pelvic exam every year to look for any signs of cancer. Any vaginal bleeding, other than menstrual bleeding, should be reported to a doctor as soon as possible.
- Hormone therapy with a luteinizing hormone-releasing hormone (LHRH) agonist is given to some premenopausal women who have just been diagnosed with hormone receptor-positive breast cancer. LHRH agonists decrease the body’s estrogen and progesterone.
- Hormone therapy with an aromatase inhibitor is given to some postmenopausal women who have hormone receptor-positive breast cancer. Aromatase inhibitors decrease the body’s estrogen by blocking an enzyme called aromatase from turning androgen into estrogen. Anastrozole, letrozole, and exemestane are types of aromatase inhibitors.
- For the treatment of early localized breast cancer that can be removed by surgery, certain aromatase inhibitors may be used as adjuvant therapy instead of tamoxifen or after 2 to 3 years of tamoxifen use. For the treatment of metastatic breast cancer, aromatase inhibitors are being tested in clinical trials to compare them to hormone therapy with tamoxifen.
- In women with hormone receptor-positive breast cancer, at least 5 years of adjuvant hormone therapy reduces the risk that cancer will recur (come back).
- Other types of hormone therapy include megestrol acetate or anti-estrogen therapy such as fulvestrant.
Targeted therapy
- Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells without harming normal cells. Monoclonal antibodies, tyrosine kinase inhibitors, cyclin-dependent kinase inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and PARP inhibitors are types of targeted therapies used in the treatment of breast cancer.
- Monoclonal antibody therapy is a cancer treatment that uses antibodies made in the laboratory, from a single type of immune system cell. These antibodies can identify substances on cancer cells or normal substances that may help cancer cells grow. The antibodies attach to the substances and kill the cancer cells, block their growth, or keep them from spreading. Monoclonal antibodies are given by infusion. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells. Monoclonal antibodies may be used in combination with chemotherapy as adjuvant therapy.
Types of monoclonal antibody therapy include the following
-
Trastuzumab – is a monoclonal antibody that blocks the effects of the growth factor protein HER2, which sends growth signals to breast cancer cells. It may be used with other therapies to treat HER2 positive breast cancer.
-
Pertuzumab – is a monoclonal antibody that may be combined with trastuzumab and chemotherapy to treat breast cancer. It may be used to treat certain patients with HER2 positive breast cancer that has metastasized (spread to other parts of the body). It may also be used as neoadjuvant therapy or adjuvant therapy in certain patients with early-stage HER2 positive breast cancer.
-
Ado-trastuzumab – emtansine is a monoclonal antibody linked to an anticancer drug. This is called an antibody-drug conjugate. It is used to treat HER2 positive breast cancer that has spread to other parts of the body or recurred (come back).
Tyrosine kinase inhibitors are targeted therapy drugs that block signals needed for tumors to grow. Tyrosine kinase inhibitors may be used with other anticancer drugs as adjuvant therapy. Tyrosine kinase inhibitors include the following:
-
Lapatinib – is a tyrosine kinase inhibitor that blocks the effects of the HER2 protein and other proteins inside tumor cells. It may be used with other drugs to treat patients with HER2 positive breast cancer that has progressed after treatment with trastuzumab.
-
Neratinib – is a tyrosine kinase inhibitor that blocks the effects of the HER2 protein and other proteins inside tumor cells. It may be used to treat patients with early-stage HER2 positive breast cancer after treatment with trastuzumab.
-
Cyclin-dependent kinase inhibitors – are targeted therapy drugs that block proteins called cyclin-dependent kinases, which cause the growth of cancer cells. Cyclin-dependent kinase inhibitors include the following:
-
Palbociclib – is a cyclin-dependent kinase inhibitor used with the drug letrozole to treat breast cancer that is estrogen receptor positive and HER2 negative and has spread to other parts of the body. It is used in postmenopausal women whose cancer has not been treated with hormone therapy. Palbociclib may also be used with fulvestrant in women whose disease has gotten worse after treatment with hormone therapy.
-
Ribociclib- is a cyclin-dependent kinase inhibitor used with letrozole to treat breast cancer that is hormone receptor positive and HER2 negative and has come back or spread to other parts of the body. It is used in postmenopausal women whose cancer has not been treated with hormone therapy.
-
Abemaciclib – is a cyclin-dependent kinase inhibitor used to treat hormone receptor positive and HER2 negative breast cancer that is advanced or has spread to other parts of the body. It may be used alone or with other drugs to treat breast cancer that has gotten worse after other treatment. Mammalian target of rapamycin (mTOR) inhibitors blocks a protein called mTOR, which may keep cancer cells from growing and prevent the growth of new blood vessels that tumors need to grow. mTOR inhibitors include the following:
-
Everolimus – is an mTOR inhibitor used in postmenopausal women with advanced hormone receptor-positive breast cancer that is also HER2 negative and has not gotten better with other treatment.
-
PARP inhibitors – are a type of targeted therapy that block DNA repair and may cause cancer cells to die. Olaparib is a PARP inhibitor used to treat patients with mutations in the BRCA1 or BRCA2 genes and HER2 negative breast cancer that has spread to other parts of the body. PARP inhibitor therapy is being studied for the treatment of patients with triple negative breast cancer.
Treatment for breast cancer may cause side effects
For information about side effects that begin during treatment for cancer, see our Side Effects page. Some treatments for breast cancer may cause side effects that continue or appear months or years after treatment has ended. These are called late effects. Late effects of radiation therapy are not common, but may include:
-
Inflammation of the lung after radiation therapy to the breast, especially when chemotherapy is given at the same time.
-
Arm lymphedema, especially when radiation therapy is given after lymph node dissection.
-
In women younger than 45 years who receive radiation therapy to the chest wall after mastectomy, there may be a higher risk of developing breast cancer in the other breast.
Late effects of chemotherapy depend on the drugs used, but may include:
-
Heart failure.
-
Blood clots.
-
Premature menopause.
-
Second cancer, such as leukemia.
Late effects of targeted therapy with trastuzumab, lapatinib, or pertuzumab may include:
-
Heart problems such as heart failure.
Patients may want to think about taking part in a clinical trial.
- For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment. Many of today’s standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
- Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
- Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring(coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
- Some of the tests that were done to diagnose cancer or to find out the stage of cancer may be repeated. Some tests will be repeated in order to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
- Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if cancer has recurred (come back). These tests are sometimes called follow-up tests or check-ups.
Early, Localized, or Operable Breast Cancer
Surgery
-
Breast-conserving surgery and sentinel lymph node biopsy. If cancer is found in the lymph nodes, a lymph node dissection may be done.
-
Modified radical mastectomy. Breast reconstruction surgery may also be done.
-
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Locally Advanced or Inflammatory Breast Cancer
Treatment of locally advanced or inflammatory breast cancer is a combination of therapies that may include the following:
-
Surgery (breast-conserving surgery or total mastectomy) with lymph node dissection.
-
Chemotherapy before and/or after surgery.
-
Radiation therapy after surgery.
-
Hormone therapy after surgery for tumors that are estrogen receptor positive or estrogen receptor unknown.
-
Clinical trials testing new anticancer drugs, new drug combinations, and new ways of giving treatment.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Locoregional Recurrent Breast Cancer
Treatment of locoregional recurrent breast cancer (cancer that has come back after treatment in the breast, in the chest wall, or in nearby lymph nodes), may include the following:
-
Chemotherapy.
-
Hormone therapy for tumors that are hormone receptor positive.
-
Radiation therapy.
-
Surgery.
-
Targeted therapy (trastuzumab).
-
A clinical trial of a new treatment.
See the Metastatic Breast Cancer section for information about treatment options for breast cancer that has spread to parts of the body outside the breast, chest wall, or nearby lymph nodes.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General informationabout clinical trials is also available.
Metastatic Breast Cancer
Treatment options for metastatic breast cancer (cancer that has spread to distant parts of the body) may include the following:
Hormone therapy
In postmenopausal women who have just been diagnosed with metastatic breast cancer that is hormone receptor positive or if the hormone receptor status is not known, treatment may include:
-
Tamoxifen therapy.
-
Aromatase inhibitor therapy (anastrozole, letrozole, or exemestane). Sometimes cyclin-dependent kinase inhibitor therapy (palbociclib, palbociclib, or abemaciclib) is also given.
-
Tamoxifen, an LHRH agonist, or both.
-
In women whose tumors are hormone receptor positive or hormone receptor unknown, with spread to the bone or soft tissue only, and who have been treated with tamoxifen, treatment may include:
-
Aromatase inhibitor therapy.
-
Other hormone therapy such as megestrol acetate, estrogen or androgen therapy, or anti-estrogen therapy such as fulvestrant.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Current Research
Human Antimicrobial Protein hCAP18/LL-37 Promotes a Metastatic Phenotype in Breast Cancer
- Human cathelicidin antimicrobial protein, hCAP18, and its C-terminal peptide LL-37 is a multifunctional protein. In addition to being important in antimicrobial defense, it induces chemotaxis, stimulates angiogenesis and promotes tissue repair. We previously showed that human breast cancer cells express high amounts of hCAP18, and hypothesised that hCAP18/LL-37 may be involved in tumor progression[rx].
Genetic variation in stromal proteins decorin and lumican with breast cancer: investigations in two case-control studies
- The stroma is the supportive framework of biologic tissue in the breast, consisting of various proteins such as the proteoglycans, decorin and lumican. Altered expression of decorin and lumican is associated with breast tumors. We hypothesized that genetic variation in the decorin (DCN) and lumican (LUM) genes may contribute to breast cancer[rx].
Prognostic impact of tumor-specific HMG-CoA reductase expression in primary breast cancer
- It has been previously reported that the tumor-specific expression of the rate-limiting enzyme, 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMG-CoAR), in the mevalonate pathway is associated with more favorable tumor parameters in breast cancer. In the present study, it is examined the prognostic value of HMG–coexpression in a large cohort of primary breast cancer patients with long-term follow up[rx].
Monoclonal antibodies in human cancer
- Mouse, chimeric, humanized and human monoclonal antibodies (MABs) are all in use for treatment of human cancer. Antibodies can activate the immune system (antibody-dependent cellular cytotoxicity [ADCC], complement-dependent cytotoxicity [CDC], induction of tumor immunity [idiotype network]). The best therapeutic effect may be obtained if MABs are used early in the course of the disease[rx].
To Learn More About Breast Cancer
For more information from the National Cancer Institute about breast cancer, see the following:
For general cancer information and other resources from the National Cancer Institute, see the following:
References
[bg_collapse view=”button-orange” color=”#4a4949″ expand_text=”Show More” collapse_text=”Show Less” ]
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4127601/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4589089/
- https://www.ncbi.nlm.nih.gov/books/NBK343636/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5715522/
- https://www.cancer.gov/about-cancer/treatment/drugs/breast
- https://www.ncbi.nlm.nih.gov/books/NBK65969/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5319297/
- https://www.ncbi.nlm.nih.gov/books/NBK65744/
- https://www.ncbi.nlm.nih.gov/books/NBK482286/
- https://www.ncbi.nlm.nih.gov/books/NBK482286/
- https://www.ncbi.nlm.nih.gov/books/NBK65973/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4491690/
[/bg_collapse]




Visitor Rating: 5 Stars
Visitor Rating: 5 Stars