Ischemic Necrosis of Bone /Osteonecrosis of the hip commonly known as avascular necrosis (AVN) of the hip, is the death of the femoral head as a result of vascular disruption. AVN of the hip results in pain around the hip which is insidious in onset. The cause is generally multifactorial and more commonly seen in males compared to females. Furthermore, the age of presentation from symptomatic AVN of the hip is younger than that of osteoarthritis. The treatment of AVN of the hip is controversial, and as such, there are many different treatment options for AVN. The ideal treatment option depends on the severity and stage of the disease.
Osteonecrosis is a degenerative bone condition characterized by the death of cellular components of the bone secondary to an interruption of the subchondral blood supply.[rx] Also known as avascular necrosis, it typically affects the epiphysis of long bones at weight-bearing joints. Advanced disease may result in the subchondral collapse which threatens the viability of the joint involved. Therefore, early recognition and treatment of osteonecrosis are essential.[rx] This activity discusses the etiology and pathogenesis of the disease, presentation, and treatment options of the most common forms of osteonecrosis.
Synonyms of Osteonecrosis
- aseptic necrosis
- avascular necrosis of bone
- ischemic necrosis of bone

Types of Avascular Necrosis (AVN)
Osteonecrosis of the Hip
- Femoral head osteonecrosis falls into two classes: traumatic or atraumatic. Of the atraumatic cases, up to 70% may be bilateral.[rx] Common classifications that map the phases of osteonecrosis of the hip include the Fiat and Arlet classification and the Steinberg classification.[rx][rx] Fiat and Arlet describe the four stages of disease progression based on clinical and radiographic findings. Stage 1 is the initiation of the disease without radiological findings. Stage IV is the end-stage with femoral head collapse, flattening, and narrowing of the joint space. The Steinberg classification incorporates the use of MRI to detect a pre-clinical lesion and also to assess the size of the lesion.
Osteonecrosis of the Knee
- Spontaneous osteonecrosis of the knee (SONK) is the most common type.[rx] Secondary osteonecrosis is commoner in a younger population and often associated with a number of risk factors common to all kinds of osteonecrosis as previously discussed. The third and rarest type is called post arthroscopic osteonecrosis and has been seen in 4% of patients having undergone an arthroscopic meniscectomy.
- SONK typically presents in the sixth decade of life and is more common in the female population. Classically it affects the medial femoral condyle, and subsequent cadaveric studies have demonstrated a watershed area of the medial femoral condyle.[rx]
Osteonecrosis of the Shoulder
- Osteonecrosis of the shoulder most frequently results from trauma however it can arise from the causes outlined above, for instance, prolonged high-dose corticosteroid usage. Most of those fracture patterns with an increased risk of avascular necrosis had an anatomic neck component. Interestingly fracture-dislocations and degree of displacement of the fragments do not predict an increased incidence of avascular necrosis of the humeral head although contradictory evidence does exist.
Osteonecrosis of the Talus
- Most commonly caused by trauma resulting in displaced fractures to the neck of the talus. The incidence of avascular necrosis increases with co-existing dislocation at the ankle joint or subtalar joint. The Hawkins classification best describes this relationship. If osteonecrosis is to occur the pathognomonic subchondral lucency known as Hawkins sign will be absent on plain radiographs at 6-8 weeks.[rx]
Osteonecrosis of the Lunate
- More commonly known as Keinbock’s disease involves a collapse of the lunate due to vascular insufficiency and osteonecrosis. A history of repetitive trauma, biomechanical factors related to ulna variance, and anatomic factors such as the presence of both dorsal and palmar blood supply may contribute to the risk of avascular necrosis. The Lichtman staging of Keinbock’s disease uses four stages. [rx]
Causes of Avascular Necrosis (AVN)
The widely accepted view in the literature is that a reduction in subchondral blood supply is responsible for osteonecrosis. However, there are numerous risk factors and theories on the development of this vascular impairment. Shah et al.[rx] succinctly categorizes these into six groups:
Idiopathic causes
- In a small percentage of cases mutations in the COL2A1 gene which codes for type 2 collagen production has demonstrated autosomal dominant inheritance patterns.[rx]
- However, in many cases, a cause cannot be identified, and these patients receive the designation of idiopathic osteonecrosis.
Direct cellular toxicity
-
Chemotherapy
-
Radiotherapy
-
Thermal injury
-
Smoking
Extraosseous arterial fracture
-
Hip dislocation
-
Femoral neck fracture
-
Iatrogenic post surgery
-
Congenital arterial abnormalities
Extraosseous venous
-
Venous abnormalities
-
Venous stasis
Intraosseous extravascular compression
-
Hemorrhage
-
Elevated bone marrow pressure
-
Fatty infiltration of bone marrow due to prolonged high-dose corticosteroid use
-
Cellular hypertrophy and marrow infiltration (Gaucher’s disease)
-
Bone marrow edema
-
Displaced fractures
Intraosseous intravascular occlusion
-
Coagulation disorders such as thrombophilias and hypofibrinolysis
-
Sickle cell crises
-
Multifactorial
Traumatic-associated risk factors
- Femoral neck fracture
- Dislocation or fracture-dislocation
- Sickle cell disease
- Hemoglobinopathies
- Caisson disease (dysbarism)
- Gaucher disease
- Radiation
Atraumatic-associated risk factors
- Corticosteroid administration
- Alcohol use
- Systemic lupus erthyematosus
- Cushing disease
- Hypersecretion of cortisol (rare)
- Chronic renal failure/hemodialysis
- Pancreatitis
- Pregnancy
- Hyperlipidemia
- Organ transplantation
- Intravascular coagulation
- Thrombophlebitis
- Cigarette smoking
- Hyperuricemia/gout
- HIV
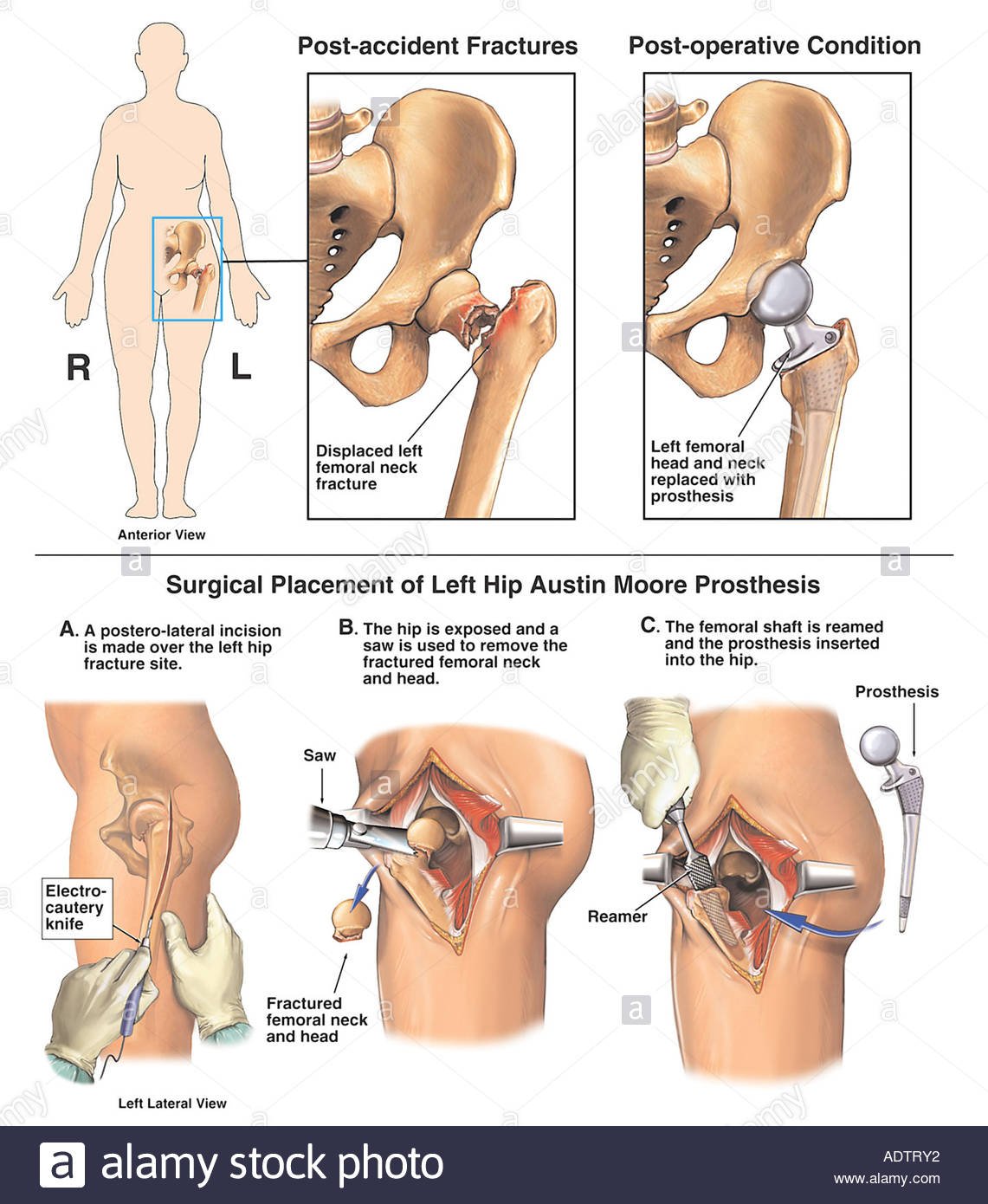
Other potential risk factors
Risk Factors
-
Irradiation
-
Trauma
-
Hematologic disease (leukemia, lymphoma)
-
Dysbaric (Caisson disease)
-
Marrow-replacing diseases (Gaucher disease)
-
Sickle cell disease
-
Alcoholism
-
Hypercoagulable states
-
Steroids
-
Systemic lupus erythematosus (SLE)
-
Transplant patient
-
Viral (CMV, hepatitis, HIV, rubella, rubeola, varicella)
-
Protease inhibitors
-
Pancreatitis
-
Vascular insult
-
Subacute bacterial endocarditis
-
Polyarteritis nodosa
-
Rheumatoid Arthritis
-
Giant cell arteritis
-
Sarcoidosis
The risk of femoral head collapse with AVN can be stratified into three groups based on the modified Kerboul combined necrotic angle. This is calculated by the summation of the arc of femoral head necrosis on mid-sagittal and midcoronal MR images.[rx]
-
Low-risk group – combined necrotic angle less than 190 degrees
-
Moderate-risk group – the combined necrotic angle between 190 and 240 degrees
-
High-risk group – combined necrotic angle greater than 240 degrees
Symptoms of Avascular Necrosis of Bone
- As the disease progresses, however, most patients experience joint pain – at first, only when putting weight on the affected joint, and then even when resting.
- Pain usually develops gradually and may be mild or severe. If osteonecrosis progresses and the bone and surrounding joint surface collapse, pain may develop or increase dramatically.
- Pain may be severe enough to cause joint stiffness by limiting the range of motion in the affected joint. Disabling osteoarthritis may develop in the affected joint.
- Pain in the joint that may increase over time and becomes severe if the bone collapses
- Pain that occurs even at rest
- Limited range of motion
- Groin pain, if the hip joint is affected
- Limping, if the condition occurs in the leg
- Difficulty with overhead movement, if the shoulder joint is affected
Diagnosis of Ischemic Necrosis of Bone
History and Physical Exam
- It is essential to obtain a full, detailed history pertaining to the symptomatology including onset, location of the pain, duration of symptoms, the characteristics of the pain, alleviating and aggravating symptoms, radiation of symptoms, and timing of symptoms. It is equally important to obtain a detailed medical and surgical history to identify any of the risk factors associated with AVN.
- Patients with AVN of the femoral head will present with symptoms of hip pain with insidious onset. Typically, the pain will be associated with standing from a seated position, stairs, inclines, and impact loading. The pain will tend to be more noticeable in the anterior hip or groin as opposed to the buttock or greater trochanter.
Radiography And Imaging
- X-Ray – An x-ray is a common tool that the doctor may use to help diagnose the cause of joint pain. It is a simple way to produce pictures of bones. The x-ray of a person with early osteonecrosis is likely to be normal because x-rays are not sensitive enough to detect the bone changes in the early stages of the disease. X-rays can show bone damage in the later stages, and once the diagnosis is made, they are often used to monitor the course of the condition.
- Bone Scan – Also known as bone scintigraphy, bone scans should not be used for the diagnosis of osteonecrosis because they may miss 20 to 40% of the bone locations affected.
- Computed/Computerized Tomography (CT) – A CT scan is an imaging technique that provides the doctor with a three-dimensional picture of the bone. It also shows “slices” of the bone, making the picture much clearer than x-rays and bone scans. CT scans usually do not detect early osteonecrosis as early as MRI scans but are the best way to show a crack in the bone. Occasionally it may be useful in determining the extent of bone or joint surface collapse.
- Biopsy – A biopsy is a surgical procedure in which tissue from the affected bone is removed and studied. It is rarely used for diagnosis, as the other imaging studies are usually sufficiently distinct to make the diagnosis with a high level of confidence.
- Nuclear medicine – Bone scintigraphy is also quite sensitive (~85%) and is the second option after MRI. It is a choice when multiple sites of involvement must be assessed in patients with risk factors, such as sickle cell disease. The findings are different accordingly to the time of the scan:
- early disease: often represented by a cold area likely representing the vascular interruption
- late disease: may show a “doughnut sign”: a cold spot with surrounding high uptake ring (surrounding hyperemia and adjacent synovitis)
- Magnetic Resonance Imaging (MRI) – MRI is a common method for diagnosing osteonecrosis. Unlike x-rays, bone scans, and CT (computed/computerized tomography) scans, MRI detects chemical changes in the bone marrow and can show osteonecrosis in its earliest stages before it is seen on an x-ray. MRI provides the doctor with a picture of the area affected and the bone rebuilding process. In addition, MRI may show diseased areas that are not yet causing any symptoms. An MRI uses a magnetic field and radio waves to produce cross-sectional images of organs and bodily tissues. MRI is the most sensitive (~95%) modality and demonstrates changes well before plain films changes are visible.
- reactive interface line: focal serpentine low signal line with fatty center (most common appearance and first sign on MRI)
- double line sign: T2WI serpentine peripheral/outer dark (sclerosis) and inner bright (granulation tissue) line is diagnostic (the line extends usually to the subchondral bone plate, which helps to differentiate it from subchondral fracture)
- diffuse edema: edema is not an early sign; instead, studies show that edema occurs in advanced stages and is directly correlated with pain
- rim sign: osteochondral fragmentation
- secondary degenerative change (i.e. osteoarthritis)
- on contrast-enhanced images non-viable marrow does not enhance
- in case of radiation necrosis, there is edema or fatty replacement of the adjacent bone marrow (depending on the interval between the examination and radiotherapy)
Staging
Steinberg Classification for staging AVN of the femoral head (modification of the Ficat classification)[rx][rx]
-
Stage 0 – Normal or nondiagnostic radiograph and MRI
-
Stage 1 – Normal radiograph, abnormal MRI
-
Stage 2 – Abnormal radiograph showing cystic and sclerotic changes in the femoral head, abnormal MRI
-
Stage 3 – Abnormal radiograph showing subchondral collapse, producing a crescent sign, abnormal MRI
-
Stage 4 – Abnormal radiograph showing flattening of the femoral head, abnormal MRI
-
Stage 5 – Abnormal radiograph showing joint narrowing with or without acetabular involvement, abnormal MRI
-
Stage 6 – abnormal radiograph showing advanced degenerative changes, abnormal MRI
There is no specific staging system for dysbaric osteonecrosis itself. However, the Ficat classification as below is utilized when staging osteonecrosis of the proximal femur.[rx]
-
Stage 0: Normal x-ray, normal MRI, no symptoms
-
Stage 1: Normal or minor osteopenia on x-ray, edema on MRI, increased uptake on bone scan, pain in the groin
-
Stage 2: Mixed osteopenia/sclerosis on x-ray, a defect on MRI, increased uptake on bone scan, groin pain and stiffness on exam
-
Stage 3: Presents with “crescent” sign or some cortical collapse on x-ray, MRI has same findings as x-ray, Increased uptake on bone scan, patient has pain radiating to knee and walks with a limp
-
Stage 4: X-ray shows end-stage collapse with secondary arthrosis of the hip joint, MRI shows similar findings as x-ray, bone scan shows increased uptake, patient presents with pain and a limp.
[stextbox id=’alert’]
Steinberg staging system [rx]
Stage Features
0 Normal radiograph, bone scan, and MRI
I Normal radiograph, abnormal bone scan and or magnetic resonance imaging
IA Mild (involves less than 15% of the femoral head).
IB Moderate (involves 15% to 30% of the femoral head)
IC Severe (involves over 30% of the femoral head)
II Cystic and sclerotic change of the femoral head
IIA Mild (involves less than 15% of the femoral head)
IIB Moderate (involves 15% to 30% of the femoral head)
IIC Severe (involves more than than 30% of the femoral head)
III Subchondral collapse (crescent sign) without flattening of the femoral head
IIIA Mild (involves under 15% of the femoral head)
IIIB Moderate (involves 15% to 30% of the femoral head)
IIIC Severe (involves over 30% of the femoral head)
IV Flattening of the femoral head/femoral head collapse
IVA Mild (involves under 15% of the femoral head)
IVB Moderate (involves 15% to 30% of the femoral head)
IVC Severe (involves greater than 30% of the femoral head)
V Joint space narrowing and/or acetabular changes
VA Mild
VB Moderate
VC Severe
VI Advanced degenerative joint disease
Steinberg staging system
| Stage | Features |
| 0 | Normal radiograph, bone scan and magnetic resonance imaging |
| I | Normal radiograph, abnormal bone scan and or magnetic resonance imaging |
| IA Mild (involves < 15% of femoral head) | |
| IB Moderate (involves 15% to 30% of femoral head) | |
| IC Severe (involves > 30% of femoral head) | |
| II | Cystic and sclerotic changes in the femoral head |
| IIA Mild (involves < 15% of femoral head) | |
| IIB Moderate (involves 15% to 30% of femoral head) | |
| IIC Severe (involves > 30% of femoral head) | |
| III | Subchondral collapse (crescent sign) without flattening of the femoral head |
| IIIA Mild (involves < 15% of femoral head) | |
| IIIB Moderate (involves 15% to 30% of femoral head) | |
| IIIC Severe (involves > 30% of femoral head) | |
| IV | Flattening of the femoral head/femoral head collapse |
| IVA Mild (involves < 15% of femoral head) | |
| IVB Moderate (involves 15% to 30% of femoral head) | |
| IVC Severe (involves > 30% of femoral head) | |
| V | Joint space narrowing and/or acetabular changes |
| VA Mild | |
| VB Moderate | |
| VC Severe | |
| VI | Advanced degenerative joint disease |
[/stextbox]
Treatment of Ischemic Necrosis of Bone
Aetna considers the following adjunctive treatments experimental and investigational for the treatment of avascular necrosis of any joint because the effectiveness of these approaches has not been established (not an all-inclusive list):
- Autologous bone marrow mononuclear cells/bone marrow concentrate/bone marrow stem cells
- Autologous platelet concentrate
- Bisphosphonates
- Bone morphogenic proteins (e.g., rhBMP-2)
- Demineralized bone matrix
- Erythropoietin
- Growth factors
- Laser therapy
- Mesenchymal stem cells
- Ozone therapy
- Parathyroid hormone
- Platelet-rich fibrin
- Platelet-rich plasma (PRP)
- PRP combined with stem cells
- PRP combined with mesenchymal stem cells and synthetic bone graft
- Synthetic bone graft substitute (e.g., calcium sulphate and calcium phosphate).
Nonoperative Treatment (Stages 0-2)
-
Bisphosphonates – This is largely limited to small lesions, less than 10% involvement of the femoral head, without evidence of collapse. The evidence is inconclusive on whether or not bisphosphonates prevent femoral head collapse.
-
Rest – Stay off the joint. This can help slow damage. You might need to hold back on physical activity or use crutches for several months.
-
Exercise – A physical therapist can show you the right moves to get a range of motion back in your joint.
- Electrical stimulation – An electrical current could jump-start new bone growth. Your doctor might use it during surgery or give you a special gadget for it.
- Faradic currents – Electrical currents might encourage your body to grow new bone to replace the damaged bone. Electrical stimulation can be used during surgery and applied directly to the damaged area. Or it can be administered through electrodes attached to your skin.
- Physical Therapy – Physical therapy may provide relief and alleviate some symptoms but generally will not preclude progressive hip ON from advancing to later stages.[rx] Similarly, restricting weight-bearing with the use of assistive devices such as crutches or a cane may be useful to control symptoms of pain, weakness, and antalgic gait. Physical therapy is not appropriate if the goal of treatment is to prevent the hip from requiring THA, and to date there is no evidence that weight-bearing restrictions are helpful in preventing progressive ON disease from advancing to end-stage disease.
Medications
Investigational medication options currently being used but that are not proven or reliably used to treat ON include 1) anticoagulants, 2) bisphosphonate antiresorptive agents, 3) cholesterol lowering statins, and 4) hyperbaric oxygen. If the doctor knows what’s causing your avascular necrosis, treatment will include efforts to manage it. This can include
- Nonsteroidal anti-inflammatory drugs (NSAIDs) – These will help with the pain. Medications, such as ibuprofen (Advil, Motrin IB, others) or naproxen sodium (Aleve) might help relieve the pain associated with avascular necrosis.
- Cholesterol drugs – They cut the amount of cholesterol and fat in your blood, which can help prevent the blockages that lead to AVN.
- Osteoporosis drugs – Medications, such as alendronate (Fosamax, Binosto), might slow the progression of avascular necrosis, but the evidence is mixed.
- Cholesterol-lowering drugs – Reducing the amount of cholesterol and fat in your blood might help prevent the vessel blockages that can cause avascular necrosis.
- Blood thinners – If you have a clotting disorder, blood thinners, such as warfarin (Coumadin, Jantoven), might be recommended to prevent clots in the vessels feeding your bones.
- Regenerative medicine treatment. Bone marrow aspirate and concentration is a newer procedure that might be appropriate for early-stage avascular necrosis of the hip. Stem cells are harvested from your bone marrow. During surgery, a core of dead hipbone is removed and stem cells inserted in its place, potentially allowing for the growth of new bone. More study is needed.
- Bisphosphonates – significantly reduce the incidence of the collapse of the FH in osteonecrotic hips by reducing osteoclast activity. Alendronate has been shown to prevent early collapse of the FH in Steinberg stages II and III non-traumatic ON at 24-28 mo follow up and has been reported to diminish the amount of pain at one year follow up when it is compared with placebo treatment[rx,rx]. Alendronate has been used as adjunctive therapy with surgical procedures and has been found to reduce pain and the risk of collapse in the early stages of ONFH[rx]. Evidence for prevention of THR and reduction of AVN progression still remains controversial[rx].
- Biophysical treatments include extracorporeal shockwave therapy (ESWT) – pulsed electromagnetic therapy, and hyperbaric oxygen (HBO) therapy. ESWT has been shown to restore tissue oxygenation, reduce edema, and induce angiogenesis and may offer an alternative to the invasive modalities for FH necrosis in the earlier stages[rx,rx]. ESWT has also been associated with improvement in both pain and function and has been found to result in a reduction of lesion size and bone marrow edema at 1-year follow up. Long term (8-9 years) improvement in pain and Harris Hip scores has also been demonstrated in the ESWT group treatment when compared with the core decompression group treatment[rx].
Surgery
-
Core decompression of the femoral head with or without stem cell injection
-
Vascularized free-fibula grafting
-
Total hip resurfacing
-
Total hip arthroplasty
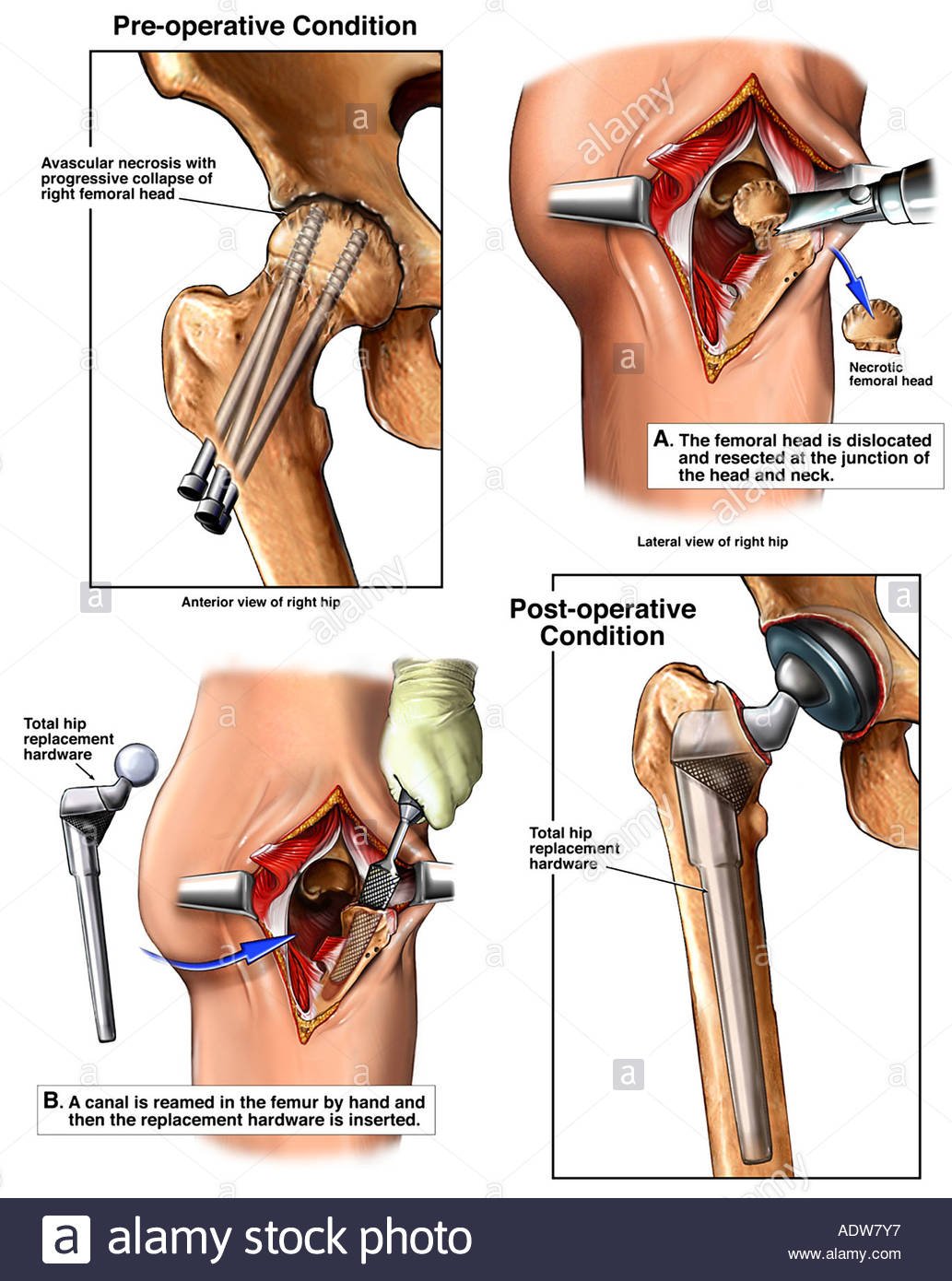
Surgical Options in Early-Stage Hip Osteonecrosis
Core Decompression
- Core decompression is a minimally invasive surgical technique performed to manage symptoms in early stages (precollapse) of the condition (eg, Ficat and Arlet Stages I and II). The procedure involves drilling holes into the femoral head to relieve pressure and create channels for new blood vessels to nourish the affected areas. The published success rates of core decompression vary greatly from 40% to 100%, depending on patient population.[rx]
- Higher success rates after core decompression are seen in patients with the earliest disease stages. Patients with successful core decompression procedures typically return to unassisted ambulation after several months and can have complete pain relief.[rx]
Bone grafting procedures
- Non-vascularized bone grafts from different sources (allograft, autograft or artificial) have been used to fill the necrotic area in the FH. The grafting can be performed through the core decompression tract, which is the most common technique, but also through a window in the FH or in the femoral neck[rx].
- This latter technique, also referred to as the trapdoor procedure, requires a surgical dislocation of the hip in order to graft the defect through a cartilage window in the FH.A standard technique uses an autograft that involves taking bone from one part of the body and moving it to another part of the body. A bone graft that is harvested from a donor or cadaver is called an allograft and is typically acquired through a bone bank.
Bone Marrow Aspirate Concentration
- The bone marrow aspirate concentration injection procedure with core decompression involves the use of concentrated bone marrow that is injected into the dead bone of the hip. This investigational technique harvests stem cells from a patient’s bone marrow and injects them into the area of ON.[rx]
- The bone marrow aspirate concentration procedure is hypothesized to prevent further progression of the disease and to stimulate new bone growth.[rx]
Percutaneous Drilling
- Another surgical option is percutaneous drilling. In this procedure, a hole is drilled percutaneously through the femoral neck to the affected site in the femoral head. One report on 45 hips with a mean follow-up of 24 months reported 24 (80%) of 30 hips with Ficat and Arlet Stage I disease had successful outcomes (defined as Harris Hip Score < 70).[rx] A more recent study comparing multiple drilling vs standard core decompression showed favorable results in favor of percutanteous drilling.[rx]
Surgical Options in Advanced-Stage Hip Osteonecrosis
Vascularized Bone Graft
- A vascularized fibula graft is a more involved surgical procedure in which a segment of bone is taken from the fibula with its blood supply. The graft is then transplanted into a hole created in the femoral neck and head, and the artery and vein are reattached to help heal the area of ON.[rx]
Osteotomy
- Osteotomy in hip ON can be performed to remove necrotic bone away from primary weight-bearing areas. Although this operation may delay THA surgery, it is most useful in patients with idiopathic ON who demonstrate small precollapse or early postcollapse of the femoral head.
- A consequence of osteotomies, however, is that they make subsequent THA more challenging and are often associated with an increased risk of nonunion of the bone.
Nonvascularized Bone Graft
- There are 3 types of nonvascularized bone grafting surgeries: 1) trapdoor procedure, 2) lightbulb technique, and 3) Phemister technique. The trapdoor procedure is one in which autogenous cancellous and cortical bone grafting have been successful in Ficat and Arlet Stage III hip ON in patients with small- to medium-sized lesions.
- A review of the results of 30 trapdoor operations performed on 23 patients with Ficat and Arlet Stage III or Stage IV ON of the femoral head performed through a so-called trapdoor made in the femoral head revealed a good or excellent result as determined by the Harris Hip Score system.[rx]
Lightbulb Technique
- The lightbulb technique uses a cortical window in the anterior aspect of the femoral neck. Necrotic bone can be removed using this window, which can be later packed with nonvascularized bone graft. Wang et al[rx] evaluated 110 patients (138 hips) who underwent the lightbulb procedure.
- At mean follow-up of 25 months, mean Harris Hip Scores improved from 62 to 79 points. A total of 94 hips (68%) were considered to have successful outcomes at latest follow-up. Radiographic improvements were noted in 100% of Association Research Circulation Osseous Stage IIa patients, 77% in stage IIb patients, and 51% in stage IIc and IIIa patients.[rx]
Phemister Technique
- In the Phemister technique, a trephine is inserted through the femoral neck to create a tract to the lesion. A second trephine is then inserted to create another tract to the lesion site. A cortical strut graft can then be placed in the lesion. A recent review reports this procedure to have a clinical success rate ranging from 36% to 90%.25
Total Hip Arthroplasty
- Once the femoral head has undergone major collapse, replacing the hip joint is the only practical operative option and offers the most predictable pain relief in advanced ON. THA is successful in relieving pain and restoring function in the majority of patients.[rx–rx] In THA, the diseased cartilage and bone constituting the hip joint is replaced with artificial implants made of metal and plastic.
Biological agents
- There is considerable enthusiasm in the development of biological therapies that can enhance core decompression with osteogenic (mesenchymal stem cells) and/or osteoinductive agents (bone morphogenic protein) that have the potential to produce better results for larger lesions.
- It has been hypothesized that there is an insufficient supply of progenitor cells in patients with AVN, which are required to enhance remodeling in areas of ON[rx].
Tantalum implants
- Porous tantalum implants in combination with core decompression offers the advantage of providing structural support without the risk of autograft harvest or the infectious complications of bone allograft[rx–rx]. Veillete et al[rx] reported an overall survival rate of 91.8% at twenty-four months, and 68.1% at forty-eight months after evaluating fifty-four patients with ONFH treated with core decompression and the insertion of a porous tantalum rod.
FHRP
Although FHSP may provide good clinical results in patients with small pre-collapse lesions, these interventions are less predictable in patients with larger lesions or in FH collapse. These patients are therefore better candidates for FHRP.
Hemi-resurfacing arthroplasty and hemipolar/bipolar hip replacement
- Hemi-resurfacing arthroplasty is a significant treatment option when the joint surface is still preserved and the articular cartilage is minimally damaged. Possible indications include a Ficat III, early stage Ficat IV, or early failure of a free vascularized fibula graft. With good patient selection and surgical technique this procedure can restore patient function although pain relief may not be as predictable as after THR[rx].
- Hemi-resurfacing arthroplasty causes little distortion of the anatomy, preserves bone, and produces minimal particle debris. Accurate evaluation of the acetabular articular cartilage and its longevity with this component poses a difficult challenge.
Hemi-arthroplasty
- The replacements are an alternative treatment strategy as they preserve the acetabular bone stock. The major concerns with this procedure are the incidence of protrusion and polyethylene wear that can lead to particle-induced osteolysis and femoral stem loosening[rx,rx]. Nevertheless, either hemi-resurfacing arthroplasty or proximal femoral osteotomies are preferred to hemi-arthroplasty.
THA
- Arthroplasty is typically reserved for patients with late-stage ONFH, as well as older patients and those with more advanced arthritis [rx]. Arthroplasty is the only treatment that has been proven to reduce pain and restore mobility. In the United States, it is estimated that approximately 10% of all THRs are done in symptomatic hip ON[rx,rx].
Prevention
To lower your risk of AVN
- Cut back on alcohol – Heavy drinking is a leading risk factor for AVN.
- Keep your cholesterol in check – Small bits of fat are the most common thing blocking blood supply to you bones.
- Use steroids carefully – Your doctor should keep tabs on you while you’re taking these medications. Let them know if you’ve used them in the past. Taking them over and over again can worsen bone damage.
- Don’t smoke – It boosts your AVN risk.
- Avoid drinking excessive amounts of alcohol.
- When possible, avoid high doses and long-term use of corticosteroids.
- Follow safety measures when diving to avoid decompression sickness.
Eponymous names for specific sites of avascular necrosis
- Ahlback disease – medial femoral condyle, i.e. SONK
- Brailsford disease – head of the radius
- Buchman disease – iliac crest
- Burns disease – distal ulna
- Caffey disease – entire carpus or intercondylar spines of the tibia
- Dias disease – trochlea of the talus
- Dietrich disease – head of metacarpals
- Freiberg infraction – head of the second metatarsal
- Friedrich disease – medial clavicle
- Hass disease – humeral head
- Iselin disease – base of 5th metatarsal
- Kienböck disease – lunate
- Köhler disease – patella or navicular (children)
- Kümmell disease – vertebral body
- Legg-Calvé-Perthes disease – femoral head
- Mandl disease – greater trochanter
- Mauclaire disease – metacarpal heads
- Milch disease – ischial apophysis
- Mueller-Weiss disease – navicular (adult)
- Panner disease – capitellum of the humerus
- Pierson disease – symphysis pubis
- Preiser disease – scaphoid
- Sever disease – calcaneal epiphysis
- Siffert-Arkin disease – distal tibia
- Thiemann disease – base of phalanges
- van Neck-Odelberg disease – ischiopubic synchondrosis
References
[bg_collapse view=”button-orange” color=”#4a4949″ expand_text=”Show More” collapse_text=”Show Less” ]
- https://www.ncbi.nlm.nih.gov/books/NBK499954/
- https://www.ncbi.nlm.nih.gov/books/NBK537007/
- https://www.ncbi.nlm.nih.gov/books/NBK546658/
- https://www.ncbi.nlm.nih.gov/books/NBK482310/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4573503/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6197539/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4292325/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6380478/
- https://www.ncbi.nlm.nih.gov/books/NBK482310/
- https://www.ncbi.nlm.nih.gov/books/NBK546658/
- https://www.ncbi.nlm.nih.gov/books/NBK537007/
- https://medlineplus.gov/osteonecrosis.html
- https://en.wikipedia.org/wiki/Spontaneous_osteonecrosis_of_the_knee
- https://en.wikipedia.org/wiki/Avascular_necrosis
[/bg_collapse]