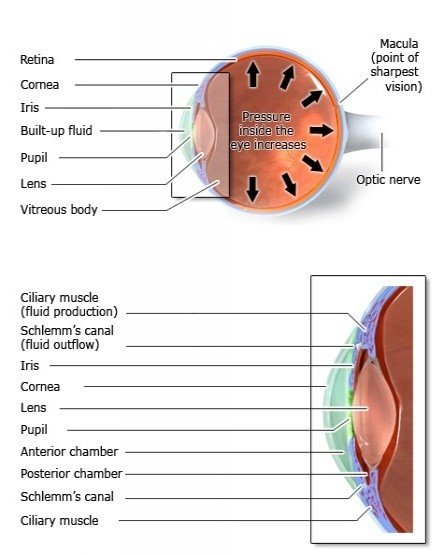
Primary Open-Angle Glaucoma/Glaucoma is a chronic, degenerative optic neuropathy that can be distinguished from most other forms of acquired optic neuropathy by the characteristic appearance of the optic nerve. In glaucoma, the neuroretinal rim of the optic nerve becomes progressively thinner, thereby enlarging the optic-nerve cup. This phenomenon is referred to as optic-nerve cupping. Its cause is the loss of retinal ganglion cell axons, along with supporting glia and vasculature. The remaining neuroretinal rim retains its normal pink color. In other optic neuropathies, the optic-nerve tissue loses its pink color and cupping does not develop. A rare exception is arteritic anterior ischemic optic neuropathy, in which cupping can occur.[rx] Patients with glaucoma typically lose peripheral vision and may lose all vision if not treated.
Glaucoma is the second leading cause of permanent blindness in the United States and occurs most often in older adults.[rx] There are four general categories of adult glaucoma: primary open-angle and angle-closure, and the secondary open and angle closure glaucoma. The most common type in the United States is primary, open-angle glaucoma (POAG).[rx] Glaucoma is defined as an acquired loss of retinal ganglion cells and axons within the optic nerve or optic neuropathy, that results in a characteristic optic nerve head appearance and a corresponding progressive loss of vision. This pattern of peripheral loss of vision can also be a distinguishing characteristic from other forms of vision loss.[rx]
Based on the IOP response to topical administration of betamethasone and dexamethasone, Armaly and Becker suggested three categories:
-
High responders (4-6% of population) – developed an IOP greater than 31 mm Hg or a rise of more than 15 mm Hg from baseline.
-
Moderate responders (about 1/3 of the population)- developed an IOP between 25-31 mm Hg orba rise of 6-15 mm Hg from baseline.
-
Non responders (about 2/3 of the population) – found to have an IOP less than 20 mm Hg or a rise of less than 6 mm Hg from baseline.
Types of Glaucoma
Glaucoma has been classified into specific types:[rx]
Primary glaucoma and its variants [Primary glaucoma (H40.1-H40.2)]
Primary open-angle glaucoma, also known as chronic open-angle glaucoma, chronic simple glaucoma, glaucoma simplex
-
- High-tension glaucoma
- Low-tension glaucoma
Primary angle closure glaucoma, also known as primary closed-angle glaucoma, narrow-angle glaucoma, pupil-block glaucoma, acute congestive glaucoma
-
- Acute angle closure glaucoma (aka AACG)[rx]
- Chronic angle closure glaucoma
- Intermittent angle closure glaucoma
- Superimposed on chronic open-angle closure glaucoma (“combined mechanism” – uncommon)
Variants of primary glaucoma
- Pigmentary glaucoma
- Exfoliation glaucoma, also known as pseudoexfoliative glaucoma or glaucoma capsulare
- Primary juvenile glaucoma
Developmental glaucoma
Developmental glaucoma (Q15.0)
- Primary congenital glaucoma
- Infantile glaucoma
- Glaucoma associated with hereditary or familial diseases
Secondary glaucoma
Secondary glaucoma (H40.3-H40.6)
Inflammatory glaucoma
-
- Uveitis of all types
- Fuchs heterochromic iridocyclitis
Phacogenic glaucoma
-
- Angle-closure glaucoma with mature cataract
- Phacoanaphylactic glaucoma secondary to rupture of lens capsule
- Phacolytic glaucoma due to phacotoxic meshwork blockage
- Subluxation of lens
Glaucoma secondary to intraocular hemorrhage
-
- Hyphema
- Hemolytic glaucoma, also known as erythroclastic glaucoma
Traumatic glaucoma
-
- Angle recession glaucoma: Traumatic recession on anterior chamber angle
- Postsurgical glaucoma
-
- Aphakic pupillary block
- Ciliary block glaucoma
Drug-induced glaucoma
-
- Corticosteroid induced glaucoma
- Alpha-chymotrypsin glaucoma. Postoperative ocular hypertension from use of alpha chymotrypsin.
Glaucoma of miscellaneous origin
-
- Associated with intraocular tumors
- Associated with retinal detachments
- Secondary to severe chemical burns of the eye
- Associated with essential iris atrophy
- Toxic glaucoma
Neovascular glaucoma
- An uncommon type of glaucoma, is difficult or nearly impossible to treat, and is often caused by proliferative diabetic retinopathy (PDR) or central retinal vein occlusion (CRVO). It may also be triggered by other conditions that result in ischemia of the retina or ciliary body. Individuals with poor blood flow to the eye are highly at risk for this condition.
Toxic glaucoma
- It is open-angle glaucoma with an unexplained significant rise of intraocular pressure following unknown pathogenesis. Intraocular pressure can sometimes reach 80 mmHg (11 kPa). It characteristically manifests as ciliary body inflammation and massive trabecular oedema that sometimes extends to Schlemm’s canal. This condition is differentiated from malignant glaucoma by the presence of a deep and clear anterior chamber and a lack of aqueous misdirection
Normal-tension or low-tension glaucoma.
- In this form of glaucoma, eye pressure remains within the “normal” range, but the optic nerve is damaged nevertheless. It is not known why this happens.
Absolute glaucoma
- Absolute glaucoma (H44.5) is the end stage of all types of glaucoma. The eye has no vision, absence of pupillary light reflex and pupillary response, and has a stony appearance. Severe pain is present in the eye. The treatment of absolute glaucoma is a destructive procedure like cyclocryoapplication, cyclophotocoagulation, or injection of 99% alcohol.
[dropshadowbox align=”none” effect=”lifted-both” width=”auto” height=”” background_color=”#ffffff” border_width=”4″ border_color=”#dddddd” ]
Classification of primary angle closure (PAC)
| (1) Primary angle closure suspect |
|
| (2) Primary angle closure (PAC) |
|
| (3) Primary angle closure glaucoma (PACG) |
|
In epidemiological research this has most often been defined as an angle in which ≥270° of the posterior trabecular meshwork (the part which is often pigmented) cannot be seen. This definition is arbitrary and its evaluation in longitudinal study is an important priority. Producing a more evidence based definition of this parameter is a major research priority.
[/dropshadowbox]
Pathophysiology
The exact cause of neuropathy encountered in open-angle glaucoma is not well known, though multiple risk factors have been identified.
These include
-
Old age
-
Race– The prevalence of OAG is three times higher in African-Americans compared to non-Hispanic whites in the USA. The prevalence of OAG has been noted to be high in Afro-Caribbean, West African and people of Latino Hispanic origin.
-
Family history- The Rotterdam Eye study found 9.2 times higher risk of having OAG if first degree relatives had glaucoma.[rx]
-
Elevated IOP
-
Myopia
-
Increased cup to disc ratio
-
Thin central corneal thickness
-
Low ocular perfusion pressure
-
Low blood pressure (systolic and diastolic)[rx]
-
High blood pressure (systemic arterial hypertension)- Several studies have shown an association of hypertension with OAG, while others have not found an association.[rx]
-
High pattern standard deviation (PSD) on visual fields
-
Low intracranial (cerebrospinal fluid) pressure
-
Other risk factors include smoking, obesity, alcohol, anxiety, stress, and sleep apnea
Elevated Intraocular pressure
- Of these risk factors, the most studied risk factor had been elevated IOP, as it is modifiable. It has been shown that once IOP rises above 21 mmHg, there is a significant increase in the risk of developing visual field loss (even with only small increases in IOP), especially once IOP rises above 26 mmHg to 30 mmHg.
- High IOP is an important risk factor for the progression of glaucoma. High fluctuation of IOP may also lead to glaucoma progression. Reduction of IOP leads to less progression or stabilization of the glaucomatous optic nerve changes and visual field changes. About 40-50% of all OAG cases have IOP below 22 mm Hg in a single screening.[rx][rx]
Pathomechanism of glaucoma
- The two main proposed mechanisms by which an elevated IOP is thought to contribute to glaucomatous damage includes vascular dysfunction resulting in ischemia to the optic nerve, and mechanical dysfunction as a result of compression of the axons by the cribriform plate.
Causes of Glaucoma
Glaucoma is often caused by increased intraocular pressure. Intraocular pressure arises in the chambers of the eye between the cornea and the lens. The chambers contain a fluid called aqueous humor that is produced by the eye itself. It flows continuously from the back (posterior) chamber to the front (anterior) chamber and then drains out through a very thin tube (Schlemm’s canal). This cycle helps to maintain a constant healthy pressure in the eye. The aqueous humor also provides nutrients to the cornea, the iris and the lens.
Risk factors for POAG [rx, rx, rx]
Race
- African people have a prevalence up to 5 times higher than other ethnic groups
- Hispanic people have a more pronounced increase with age
Age
- There is an exponential increase with increased age
Refractive error
- There is an increased risk with high refractive error, both myopia and hypermetropia
Central corneal thickness
- There is an increased risk with thin central corneal thickness
Optic disc diameter
- There is an increased risk with a large optic disc diameter
Intraocular pressure
- There is an increased risk for onset and progression with elevated IOP and a decreased risk for progression with lowering of IOP
Blood pressure
- There is less risk in young persons with hypertension and increased risk in older persons with hypertension.
Cardiovascular disease
- Cardiovascular disease and glaucoma are probably closely related.
Hypothyroidism
- Thyroid disorders may increase the risk of glaucoma
Physical activity
- There is an increased risk for low ocular perfusion pressure with low physical activity
Risk factors for development of PAC [rx]
-
Axial hyperopia
-
Family history of angle closure
-
Advancing age
-
Female gender
-
East Asian ethnicity
-
Inuit ethnicity
-
Latino ethnicity
-
Shallow peripheral anterior chamber
-
Short axial length eyes
Systemic hypotension and hypertension
- Systemic hypertension, vasospasm, and acute hypotension have been proposed as potential risk factors for glaucoma in clinic-based studies.[rx], rx, rx] Several studies have reported associations between low diastolic pressure, lower ocular perfusion pressure (OPP) and higher prevalence and/or incidence of glaucoma.[rx, rx,rx, rx]
Vasospasm
- Vasospasm represents vascular dysregulation associated with inappropriate constriction or insufficient dilatation in the microcirculation.[rx] The eye is frequently involved in a vasospastic syndrome with vasospasm associated with anterior ischaemic optic neuropathy and glaucoma.[rx]
- Vasospasm is often falsely equated with Raynaud’s phenomenon.[rx] Vasospasm patients often present with cold hand symptoms but they usually do not have the pale fingers which are characteristic of Raynaud’s disease.[rx] Vasospasm patients frequently have low BP[rx] which, as discussed, may also be associated with reduced OPP and glaucoma risk.
Migraine
Pigmentary dispersion syndrome
- Pigmentary glaucoma characteristically develops in young myopic patients with pigmentary dispersion syndrome.[rx] Male gender, black race, severe myopia, and Krukenberg spindles were identified as possible risk factors for the development and severity of glaucoma in the pigment dispersion syndrome.[rx]
Pseudoexfoliation syndrome
Obstructive sleep apnea syndrome
- Compared to normal patients, obstructive sleep apnea syndrome patients were found to have 1.67 times greater likelihood of developing glaucoma over a 5 year follow-up period.[rx]
Diabetes
- A meta-analysis by Zhou and co-authors found that six case-control studies indicated diabetes as a risk factor for POAG with a mean odds ratio greater than one, while a seventh study found an odds ratio of 0.61.[rx]
- Of six population-based cohort studies five indicated a significant association between diabetes mellitus and POAG.[rx] It appears that diabetes may increase the risk of POAG, especially as hyperglycaemia results in heightened sensitivity to IOP and risk of neuronal injury.[rx]
Symptoms of Glaucoma
- Peripheral vision is gradually lost. This nearly always affects both eyes.
- In advanced stages, the patient has tunnel vision.
- Eye pain is often accompanied by nausea and sometimes vomiting.
- Lights appear to have extra halo-like glows around them.
- Seeing halos around lights
- Vision loss
- Redness in the eye
- Eye that looks hazy (particularly in infants)
- Nausea or vomiting
- Eye pain
- Narrowed vision (tunnel vision)
- Patchy blind spots in your side (peripheral) or central vision, frequently in both eyes
- Tunnel vision in the advanced stages
- Severe headache
- Blurred vision
- Halos around lights
- Eye redness
Diagnosis of Glaucoma
History and Physical
Early changes in vision due to open-angle glaucoma involves a loss of peripheral vision at first, while visual acuity (e.g., central vision) is maintained until late in the course of the disease. For this reason, open-angle glaucoma progresses relatively asymptomatically, and patients often do not present with symptoms or visual complaints until late in the course of the disease. Thus, it is a silent, but potentially blinding disease. History should include:
-
A previous ocular history such as the history of eye pain or redness, headaches, uveitis, diabetic retinopathy, cataracts, vascular occlusions, or multicolored halos
-
Race or ethnicity
-
Refractive error
-
Chronic use of topical or systemic corticosteroids
-
Ocular surgery like photocoagulation or refractive procedures, cataract surgery, glaucoma surgery, and systemic surgery/ medications
-
Head or ocular trauma
-
Take certain medications such as hypertensive medications that may influence IOP or systemic/topical steroids
-
Medical history of diabetes mellitus, migraine headaches, hypertension, vasospasm, cardiovascular disease, breathlessness, cardiac arrhythmia
-
Family history (e.g., first-degree relative with glaucoma, especially significant if this is a sibling) that would place them at a greater risk of developing open-angle glaucoma.
-
Old medical documentation of IOP, optic disc, visual field, and others.
When examining the anterior segment of the eye via slit-lamp examination, the practitioner should also examine the following for any damage, defects or irregularities that may or may not be related to glaucomatous disease:
-
Cornea for keratic precipitates, pigmented endothelium, congenital abnormalities, guttae
-
Anterior chamber for depth, uveitis, hyphema
-
Iris for transillumination defects, atrophy, differences in coloration, ectropion uveae, iris bombe, peripheral iridectomy
-
Lens for presence or progression of cataracts
-
Fundus for disc drusen, optic pits, retinal disease (age-related macular degeneration, diabetic maculopathy, retinal vascular occlusion)
-
Optic nerve/nerve fiber layers for cup-to-disc ratio, disc appearance, nerve fiber appearance.
-
Gonioscopy for the examination of the angle of the anterior chamber
Evaluation of optic nerve
- The optic nerve should ideally be evaluated using a slit lamp and 90D or 78D lens so that the 3-dimensional features of the optic nerve is better appreciated. Normally, the inferior neuroretinal rim (NRR) is the thickest, followed by superior, nasal, and temporal NRR. This is called ISNT rule. In OAG, this rule is not followed, as superior and inferior NRR gets thinned in the disease. The optic cup should be determined by its contour and not its color.
Typical optic nerve head changes in OAG include
-
Diffuse or focal narrowing (notching/shelving) of the neuroretinal rim (NRR) specifically at the superior or inferior part of the optic disc
-
Increased vertical cup to disc ratio (CDR) and thinning of NRR
-
Asymmetry of CDR of 0.2 or more
-
Hemorrhage at or around the optic disc
-
Peripapillary atrophy
-
Baring of circumlinear vessels- There is a gap between the superficial vessels and disc margin
-
Bayonetting of vessels- The vessel first goes back and then climbs along the wall of the deep cup and then angles again on the disc margin
-
Very deep (excavated) cup with bean-pot cupping and laminar dot sign
-
Nasalization of optic disc vessels, and
-
Diffuse or focal (arcuate) thinning/defect of the retinal nerve fiber layer (RNFL) contiguous with an area of NRR-notch
-
The NRR is typically pink and not pale in OAG. Pallor of the NRR usually denotes a neurological cause (eg, pituitary tumor), but may also be seen in primary angle closure glaucoma.
Visual field
To make a diagnosis of acquired glaucomatous visual field defect Hoddap–Parrish–Anderson criteria is used:[rx]
-
Glaucoma hemifield test outside normal limits on at least 2 fields OR
-
a cluster of three or more non-edge points in a location typical for glaucoma, all of which are depressed on the pattern deviation plot at a P <5% and one of which is depressed at a P <1% on 2 consecutive fields; or
-
a corrected pattern standard deviation that occurs in less than 5% of normal fields on 2 consecutive fields.’
Typical visual field changes in OAG include
-
Increased variability of responses in an area which later developed field defects
-
Asymmetry of the visual field between the eyes
-
Paracentral scotoma- commonly superonasal
-
Roenne’s nasal step- an area of depression above or below the horizontal meridian in the nasal side
-
Temporal wedge
-
Sickle-shaped (Seidel’s) scotoma
-
Bjerrum’s scotoma or arcuate scotoma
-
Annular/ring scotoma when arcuate scotoma is present on both above and below the horizontal meridian
-
General constriction of peripheral field
-
A temporal island of the visual field
Glaucoma Testing Includes
- Patient history – to determine any symptoms the patient is experiencing and if there are any general health problems and family history that may be contributing to the problem.
- Visual field test – This test measures your peripheral (side vision). It helps your eye care professional tell if you have lost peripheral vision, a sign of glaucoma.
- Dilated eye exam – In this exam, drops are placed in your eyes to widen, or dilate, the pupils. Your eye care professional uses a special magnifying lens to examine your retina and optic nerve for signs of damage and other eye problems. After the exam, your close-up vision may remain blurred for several hours.
- Visual acuity measurements – to determine if vision is being affected.
- Tonometry – to measure the pressure inside the eye to detect increased risk factors for glaucoma.
- Pachymetry – to measure corneal thickness. People with thinner corneas are at an increased risk of developing glaucoma.
- Visual field testing – also called perimetry, to check if the field of vision has been affected by glaucoma. This test measures your side (peripheral) vision and central vision by either determining the dimmest amount of light that can be detected in various locations of vision, or by determining sensitivity to targets other than light.
- Evaluation of the retina of the eye – which may include photographs or scans of the optic nerve, to monitor any changes over time.
- Supplemental testing – which may include gonioscopy. This procedure offers a view of the angle anatomy, which is where eye fluid drainage occurs. Serial tonometry is another possible test. This procedure acquires several pressure measurements over time, looking for changes in the eye pressure throughout the day. In addition, devices can be used to measure nerve fiber thickness and to look for tissue loss on specific areas of the nerve fiber layer .
- Gonioscopy – Gonioscopy should be performed to document the status of angle and to document that the angle is open, which is an important pre-requisite for diagnosing OAG.
- Photographic documentation of the optic nerve head – is done by color and red-free disc photo preferably stereo photos. Red-free images highlight area of RNFL defects.
Automated analysis and detection of deviation from normal of the optic nerve head and retinal nerve fiber layer may be performed using various technologies including
-
Optical coherence tomography (Optovue, Cirrus, Spectralis, and others)
-
Scanning laser polarimetry (GDx-VCC)
-
Confocal scanning laser ophthalmoscopy (Heidelberg retinal tomogram or HRT II)
Differential Diagnosis
Optic nerve head diseases including
-
Physiological cup- A deep cup with a healthy neuroretinal rim, normal retinal nerve fiber layer thickness, and no visual field defect. The disc size may be large
-
Optic disc drusen
-
Optic disc coloboma
-
Anomalous optic disc
-
Tilted disc
-
Ischemic optic neuropathy
Retinal diseases causing similar visual field defects
-
Branch retinal vein occlusion
-
Branch retinal artery occlusion
-
Retinitis pigmentosa
-
Panretinal photocoagulation
Central nervous system diseases
-
Pituitary tumor- The NRR is typically pale, pallor is more than cupping, and there is bitemporal hemianopia which respects vertical line passing through the fixation (contrary to glaucoma, it which visual field respects horizontal meridian)
-
Cerebrovascular accident
-
Multiple sclerosis
Treatment of Glaucoma
The following medications are used in the form of eye drops:
-
Beta blockers – increase the flow of aqueous humor out of the eye and are often prescribed as first-line therapy.
-
Cholinergic drugs –increase the outflow of aqueous humor. This is also a common, tried and tested treatment for glaucoma.
-
Prostaglandins – increase the flow of aqueous humor out of the eye and, like beta blockers, are often prescribed as first-line therapy.
-
Alpha-adrenergic agonists (sympathomimetics) – lower the production of aqueous humor and at the same time increase its outflow.
-
Carbonic anhydrase inhibitors – lower the production of aqueous humor.
-
Medical management – medical management of this condition is similar to that of primary open angle glaucoma. The agents which may be used include beta blockers, alpha-2 agonists, and carbonic anhydrase inhibitors. Beta-blockers are the first line agents in this condition. Prostaglandin analogs are relatively contraindicated in cases of steroid-induced glaucoma following uveitis treatment.
Topical medications
Prostaglandin analog- Reduces IOP by 25% -33%. The usual dose in once daily. Side effects include lengthening of eyelashes, pigmentation of lids/ iris, exacerbation of uveitis/herpetic infection, and cystoid macular edema. It is preferred as initial therapy.
-
Latanoprost
-
Travoprost
-
Bimatoprost
-
Tafluprost
-
Latanoprostene Bunod- This molecule donates has nitric oxide-donating property.
Adrenergic agents: Reduces IOP by 20%-25%. Brimonidine may cause allergic blepharoconjunctivitis and apnea/lethargy/bradycardia in children.
-
Brimonidine
-
Apraclonidine
Beta-blockers – Reduces IOP by 20%-25%. Non-selective beta-blockers should be avoided in chronic obstructive pulmonary disease and asthma. Other contraindications include heart block, hypotension, and bradycardia.
-
Non-selective-
-
Timolol
-
-
Selective
-
Betaxolol
-
Carbonic anhydrase inhibitor: Reduces IOP by 15%-20%.[rx]
-
Dorzolamide
-
Brinzolamide
Cholinergic/parasympathomimetic agents – Reduces IOP by 20%-25%.
-
Pilocarpine
Systemic agents
These are used in the acute rise of IOP or when topical medications are not tolerated.
Carbonic anhydrase inhibitor
-
Acetazolamide
Osmotic agents
-
Mannitol
-
Glycerol
Laser trabeculoplasty
- It may be considered if medical management is unsuccessful and there is a threat of impending optic nerve damage. It may also be considered in patients experiencing undesirable side effects with antiglaucoma drugs.
The indications of laser trabeculoplasty include
-
As a primary therapy for OAG
-
To reduce the number of glaucoma drops
-
Non-compliance/intolerance to medical therapy
-
Failure of medical therapy as a less invasive alternative therapy compared to surgery
The available methods of laser trabeculoplasty are
-
ALT (Argon laser trabeculoplasty)- More than 75% of unoperated eyes have a good reduction of IOP initially. Within 5 years, 30% to >50% eyes need additional surgical management. Repeat ALT usually (90%) fails to control IOP by 2 years.
-
SLT (Selective laser trabeculoplasty)- It uses Q-switched frequency doubled Nd-YAG. The efficacy of SLT is similar to ALT.
-
Micropulse laser trabeculoplasty-
Diode laser cyclophotocoagulation (DLCP)
DLCP is a method for ablation of the ciliary processes which secrete aqueous. Indications for DLCP include:
-
Uncontrolled IOP in eyes with ambulatory vision if chances of surgical success are poor
-
Uncontrolled IOP in painful blind eyes or eyes with minimal visual potential
-
Uncontrolled IOP with maximal medication after failed glaucoma surgery/ies
Surgical Management of OAG
Indications for surgical management of glaucoma are
-
IOP above target pressure or progression of visual fields and optic disc changes despite good compliance and maximally tolerable glaucoma medication
-
To avoid excessive glaucoma drops
-
Significant barriers to effective and regular medication use including cost, compliance, physical disability, inconvenience, side effects, psychosocial
-
Primary therapy for advanced glaucoma requiring very low target IOP
-
Patient preference over other options
Surgical options include
-
Trabeculectomy- Success rate may vary from 31-88%. The success rate increases with the use of mitomycin C or 5-fluorouracil. These agents, however, increase the risk of late-onset bleb leak, hypotony, and bleb-related infection.
-
Glaucoma drainage device- Molteno, Baerveldt, Ahmed.
Non-penetrating glaucoma surgery
-
Deep sclerectomy
-
Viscocanalostomy
-
Canaloplasty
Minimally invasive or micro-invasive glaucoma surgery (MIGS)[rx]– MIGS provides is a conjunctiva-sparing surgery with an ab-interno approach to reduce IOP in mild to moderate glaucoma. The different approaches include:[rx]
-
‘increasing trabecular outflow (Trabectome, iStent, Hydrus stent, gonioscopy-assisted transluminal trabeculotomy, excimer laser trabeculotomy);
-
suprachoroidal shunts (Cypass micro-stent);
-
reducing aqueous production (endocyclophotocoagulation); and
-
subconjunctival filtration (XEN gel stent)’
Cyclocryotherapy
This is another method of cycloablation using cryotherapy usually reserved for painfully blind eyes.
- Trabeculectomy – Glaucoma surgery is done to lower intraocular pressure permanently. The most common type of surgical treatment for glaucoma is called trabeculectomy. It involves cutting out a bit of the sclera (the white outer layer of the eye) and the iris in order to allow aqueous humor to flow out of the eye more easily, thereby lowering the pressure inside the eye. Possible adverse effects of this type of surgery include vision problems immediately after surgery, scarring and – over the long term – cataracts.
- Laser therapy – Intraocular pressure can also be lowered using lasers, although this is not usually as effective as surgery. In early stages of glaucoma, laser therapy can improve the outflow of aqueous humor. Laser therapy can also be used in addition to eye drops. In later stages, a laser can sometimes be used to destroy part of the tissue that produces the aqueous humor, thereby lowering the intraocular pressure. Laser therapy can cause temporary red or dry eyes and blurry vision immediately after treatment.
[dropshadowbox align=”none” effect=”lifted-both” width=”auto” height=”” background_color=”#ffffff” border_width=”4″ border_color=”#dddddd” ]
| Drug class | Drug | Route | Purported mechanism of action | Clinical trial phase |
|---|---|---|---|---|
| NMDA receptor antagonist | Memantine | Oral | Prevents excitotoxicity and apoptosis | C |
| Bis(7)-Tacrine | PCT | |||
| Antioxidants | N-acetylcysteine | Topical | Mops up ROS | None |
| Vitamin E | ||||
| Antioxidant, anti-inflammatory, and antimicrobial Forskolin (terpenes) | Forskolin (flavonoid) | Oral | Forskolin ↓ IOP by ↑ cAMP | C |
| Rutin and vitamins B1 and B2 | All maintain retinal nerve fiber layers | |||
| Antioxidant | Forskolin, Rutin, vitamin B plus PGA or beta-blocker | Oral | Maintain retinal nerve fiber layers ↓ IOP | NYR |
| Cannabinoids | Δ-1-THC | IV/oral | Improves TM outflow | NR |
| Δ-9-THC | ||||
| Marijuana | Oral | |||
| Food additives and herbs | Vitamin, mineral, and medical herbs–Marijuana | Oral | Reverse neuropathy | I and II but T |
| Mirtogenol (Flavonoid) | Pycnogenol | Oral | Increases ocular blood flow | None |
| Mirtoselect | ||||
| Hematopoietic agent | Erythropoietin | Intraperitoneally | Neuroprotection via increased survival of RGC | PCT |
| Calcium channel blocker | Nimodipine | Oral | Neuroprotective effects on neurons undergoing apoptosis and necrosis | NR |
Abbreviations: POAG, primary open-angle glaucoma; NR, not registered; T, terminated; C, completed; NYR, not yet recruiting; PCT, preclinical trial; ROS, radical oxygen species.
[/dropshadowbox]
- Monotherapy – In general monotherapy is the first treatment approach. It increases compliance and decreases systemic and topical adverse reactions, especially if the drug in question is used or applied once daily.[rx] If the drug is not efficacious or tolerable, it should be changed.
- Brimonidine – was found to be as efficacious as timolol, if not better, in reducing IOP. Topical brimonidine and not timolol should be considered a first-line treatment in patients with hypertension and glaucoma who are on concurrent systemic beta blockers.[rx] Betaxolol, which is supposed to be an improvement on timolol, does not seem to be. Both betaxolol and timolol impair lung function and betaxolol even raises IOP.[rx] In another study involving three beta-blockers, carteolol, betaxolol, and timolol, each administered as a monotherapy, it was found that these medications did not adequately treat over 50% of enrolled patients’ eyes during the 7 years of the study
- Pilocarpine – are the ones that come close to targeting the TM, although indirectly. They should be excellent for treating PACG because they open up the drainage angle by removing the blockage caused by the covering iris. The cholinergics cause the ciliary muscle to contract, and in doing so, open up the drainage pores in the meshwork. This unique mechanism of action causes contraction of the ciliary muscle and relaxing of the lens, resulting in a more spherical lens shape.
- Memantine and bis(7)-tacrine – Some studies have drawn a link between Alzheimer’s disease, Parkinson’s, and POAG. The commonality between these conditions is the excessive production of glutamate or its accumulation resulting in excitotoxicity through overstimulation of the NMDA receptors. This results in retina ischemia.[rx,rx] Memantine is known to selectively and uncompetitively block the NMDA receptor. An analysis of the composition of vitreous fluid of humans with POAG and monkeys with POAG indicated elevated levels of glutamate.[rx]
- Mirtogenol – Studies have looked into the possibility of increasing blood supply to the optical nerves to avert ischemia. Mirtogenol, a food supplement, has been considered for use as a prophylactic measure. In a study by Steigerwalt et al, IOP and ocular blood flow through the central retinal, ophthalmic, and posterior ciliary arteries were measured in two groups of human subjects with elevated IOPs (22 to 26 mmHg) but without glaucoma, and not receiving any treatment for elevated IOP.
- Vitamin E, N-acetylcysteine, and other antioxidants – Researchers have looked into whether oxidative stress is a contributing factor in POAG progression. In a study using surgically removed and cultured TM tissue from both POAG and non-POAG patients, tissue analysis revealed higher concentrations of radical oxygen reactive species (RORS), a decreased change in membrane potential, and 30% lower ATP production in the POAG TM when compared to the non- POAG TM.[rx]
- Erythropoietin – Erythropoietin has been found to have neuroprotective effects. This was observed in a DBJ/2J mouse model of POAG, where erythropoietin was administered at doses between 3000 and 12,000 U/kg, and prevented the loss of retinal ganglion cells. This was similar to the effects of memantine. However, untreated DBJ/2J control animals had a loss of retinal ganglion cells.[rx]
- Marijuana – Marijuana has been noted to reduce IOP by increasing aqueous drainage through the uveoscleral outflow pathway. In a case report involving one patient, it acted as a last-line therapy for two reasons: the patient was clearly intolerant of other medications, and other medications were not efficacious enough. Smoking marijuana cigarettes combined with the ingestion of 1 or 2 marijuana cookies reduced IOP from 30 mmHg to 15 mmHg.[rx]
- Nimodipine – Vascular dysregulation has been implicated in POAG. One theory is that ischemia of the retina is caused by lack of adequate blood supply due to the squeeze experienced by the blood vessels serving the optic nerve and the retina as a result of the high IOP. This squeeze can also cause blood vessels to burst, resulting in hemorrhage. Calcium channel blockers are known to relieve the pressure on the blood vessels. In a study involving the use of oral nimodipine, it was found to indirectly improve color sensitivity by increasing blood flow to the optic nerve head in NTG patients[rx] and improve the visual field[rx] compared to the placebo group.
[dropshadowbox align=”none” effect=”lifted-both” width=”auto” height=”” background_color=”#ffffff” border_width=”4″ border_color=”#dddddd” ]
Classes of Medications Used to Lower Intraocular Pressure
| Class of Medication | Example | Usual Dosages | Mechanism of Action | Local Adverse Effects | Systemic Adverse Effects |
|---|---|---|---|---|---|
| Prostaglandin analogues (prostamide) | Latanoprost, travoprost, tafluprost, unoprostone, bimatoprost | 1/d At night | Increase in uveoscleral outflow of aqueous humor | Conjunctival hyperemia, lengthening and darkening of eyelashes, brown discoloration of the iris, uveitis, macular edema | Minimal systemic adverse effects; may be related to headaches |
| β-Adrenergic blockers | Timolol, levobunolol, carteolol, metipranolol, betaxolol | 1/d In the morning | Reduction of aqueous humor production | Ocular irritation and dry eyes | Contraindicated in patients with asthma, chronic pulmonary obstructive disease, and bradycardia |
| α-Adrenergic agonists | Brimonidine, apraclonidine | 3/d (Sometimes 2/d) | Initial reduction of aqueous humor production with subsequent effect of increase in outflow | Ocular irritation, dry eyes, allergic reaction is relatively common | Central nervous system effects and respiratory arrest in young children; caution in patients with cerebral or coronary insufficiency, postural hypotension, and renal or hepatic failure |
| Carbonic anhydrase inhibitors | Dorzolamide, brinzolamide, acetazolamide (oral) | 3/d (Sometimes 2/d) | Reduction of aqueous humor production | Ocular irritation, dry eyes, burning sensation with topical agents | Topical form has minimal systemic adverse effects; oral form may be associated with paresthesia, nausea, diarrhea, loss of appetite and taste, lassitude, or renal stones |
| Cholinergic agonists | Pilocarpine, carbachol | Usually 4/d, but may vary | Increase in aqueous humor outflow | Ocular irritation, induced myopia and decreased vision due to ciliary spasm | Ciliary spasm leading to |
Summary of included studies providing evidence on the effect of individual dietary components on IOP and glaucoma
| Component | Study | Design | Sample | Key finding |
|---|---|---|---|---|
| Alcohol | Ramdas et al. [rx] | Prospective cohort | 3939 healthy subjects | Incidence of POAG was not associated with alcohol consumption |
| Giurlani et al. [rx] | Non-randomized trial | 73 healthy subjects | Acute ingestion of alcohol results in a statistically significant reduction in IOP | |
| Buckingham et al. [rx] | Non-randomized trial | 6 healthy subjects | On average, the IOP decreased by 3.7 mmHg following acute ingestion of alcohol, and normal values were restored after 65 min | |
| Klein et al. [rx] | Cross-sectional | 4926 subjects | Alcohol consumption was not related to the prevalence of POAG | |
| Kang et al. [rx] | Prospective cohort | 120,379 healthy subjects | Alcohol consumption did not influence the risk of developing POAG | |
| Xu et al. [rx] | Cross-sectional | 4141 subjects | Alcohol consumption was not related to the prevalence of POAG | |
| Kahn et al. [rx] | Cross-sectional | 2433 subjects | Alcohol consumption was directly related to glaucoma | |
| Fan et al. [rx] | Case–control | 32 POAG patients, 96 controls | Alcohol consumption offers a protective effect against POAG | |
| Seddon et al. [rx] | Case–control | 100 OHT patients, 100 controls | Absence of alcohol intake was significantly associated with OHT | |
| Coffee | Ajayi et al. [rx] | Randomized controlled trial | 40 healthy subjects | A statistically significant elevation in IOP was noted following acute ingestion of caffeinated coffee |
| Jiwani et al. [rx] | Randomized controlled trial | 22 POAG, 18 NTG, 20 OHT, 21 POAG suspect, and 25 healthy individuals | A statistically significant elevation in IOP was noted following acute ingestion of caffeinated coffee when pooling all groups together | |
| Avisar et al. [rx] | Randomized controlled trial | 6 NTG patients and 22 OHT patients | A statistically significant elevation in IOP was noted following acute ingestion of regular caffeinated coffee in both groups | |
| Higginbotham et al. [rx] | Randomized controlled trial | 13 POAG or POAG suspects randomized into two groups | A higher IOP was noted upon acute ingestion of coffee, compared to tea. The difference between both interventions was statistically significant at 90 min | |
| Chandrasekaran et al. [rx] | Cross-sectional | 3654 subjects | POAG patients reporting a high intake of caffeine had a significantly higher IOP compared to those reporting no intake | |
| Kang et al. [rx] | Prospective cohort | 124,172 healthy subjects | Caffeine intake did not influence POAG risk | |
| Wu et al. [rx] | Cross-sectional | 1678 subjects | No association was found between coffee consumption and glaucoma risk | |
| Pasquale et al. [rx] | Prospective cohort | 120,179 subjects | A positive association between heavy coffee consumption and EG/EGS was noted | |
| Tea | Wu et al. [rx] | Cross-sectional | 1678 subjects | Daily consumers of hot tea are less likely to have glaucoma compared to non-consumers |
| GBE | Lee et al. [rx] | Retrospective | 42 NTG patients treated with GBE 80 mg three times daily | GBE slowed the visual field damage in patients with NTG |
| Chung et al. [rx] | Randomized controlled trial | 11 healthy subjects | GBE significantly increased ocular blood flow in healthy subjects when compared to placebo | |
| Park et al. [rx] | Randomized controlled trial | 30 NTG patients | GBE significantly increased peripapillary blood flow in patients with NTG when compared to placebo | |
| Shim et al. [rx] | Case–control | 332 NTG patients: 132 received anthocyanins, 103 received GBE, and 97 were controls | Both anthocyanins and GBE were associated with improved visual function in patients with NTG | |
| Quaranta et al. [rx] | Randomized controlled trial | 27 NTG patients with pre-existing visual field damage | GBE improved pre-existing visual field damage in patients with NTG | |
| Guo et al. [rx] | Randomized controlled trial | 28 newly diagnosed NTG patients | GBE had no effect on visual field in newly diagnosed NTG patients | |
| Fruits and vegetables | Giaconi et al. [rx] | Cross-sectional | 584 African American subjects | A decreased likelihood of glaucoma was noted among women that reported a higher intake of fruits and vegetables containing vitamins A and C, in addition to carotenes |
| Coleman et al. [rx] | Cross-sectional | 1155 female subjects | Consumption of fruits and vegetables rich in vitamin A and carotenes was associated with decreased risk of glaucoma | |
| Kang et al. [rx] | Prospective cohort | 104,987 subjects | A higher intake of nitrates and green leafy vegetables was associated with a lower risk of POAG | |
| Chocolate | Terai et al. [rx] | Randomized controlled trial | 30 glaucoma patients, 30 controls | An increase in retinal vessel diameter was noted 2 h after dark chocolate intake in healthy subjects, but not in glaucoma patients |
| Saffron | Jabbarpoor Bonyadi et al. [rx] | Randomized controlled trial | 34 POAG patients | A statistically significant ocular hypotensive effect was noted after 3 weeks of treatment with saffron extract |
POAG primary open angle glaucoma, IOP intraocular pressure, OHT ocular hypertension, NTG normal tension glaucoma, EG exfoliation glaucoma, EGS exfoliation glaucoma suspect, GBE Ginkgo biloba extract
[/dropshadowbox]
Alcohol
It has been shown that alcohol lowers IOP following acute ingestion in both glaucoma patients and healthy subjects [rx, rx]. The exact mechanism leading to such finding is poorly understood; however, it is thought to be secondary to variable physiological changes such as a hyperosmotic effect exerted by alcohol, reduction of net water movement into the eye through vasopressin suppression [rx], and inhibition of secretory cells in the ciliary processes [rx].
Coffee
Coffee is a rich source of caffeine, a biologically active compound that exerts numerous physiological effects on the human body. A transient elevation in IOP has been noted following caffeine ingestion in patients with different types of glaucoma [rx–rx] and, to a lesser extent, in healthy individuals [rx, rx]. Furthermore, the Blue Mountains Eye Study [rx] found a higher mean IOP among POAG patients that reported regular caffeine consumption.
Tea
The nutritional value of tea is derived from its major constituents (i.e. polyphenols, caffeine, and minerals) [rx]. Flavonoids, a major polyphenol in tea, are thought to play a role in glaucoma [rx, rx] owing to their various physiological actions that are proposed to affect non-IOP-dependent mechanisms. Studies have shown that flavonoids demonstrate their protective effect by reducing oxidative stress [rx] and improving blood flow [rx].
Ginkgo biloba Extract
Ginkgo biloba extract (GBE) has been tested in the treatment of various medical conditions [rx], including glaucoma [rx, rx]. GBE exhibits a myriad of pharmacological properties making it relevant to the pathogenesis of glaucoma. The current evidence suggests that GBE increases ocular blood flow [rx], improves retinal ganglion cell survival [rx], and protects against oxidative stress [rx].
Fruits and Vegetables
Being rich in antioxidants, it is speculated that a diet rich in fruits and vegetables can decrease the risk of developing glaucoma. However, the nutrient composition of different fruits and vegetables varies, making it difficult to link the presumed protective effect to a single source. Nonetheless, multiple cross-sectional studies were conducted using food frequency questionnaires in an attempt to link certain fruits and vegetables to glaucoma. [rx]
Chocolate
Dark chocolate is a rich dietary source of polyphenol compounds, specifically flavonoids [rx]. Acute consumption of dark chocolate has been shown to reverse vascular endothelial dysfunction by decreasing oxidative stress and increasing the bioavailability of NO [rx]. Therefore, it is proven beneficial in patients with certain cardiovascular diseases such as HTN [rx] and peripheral artery disease [rx].
Saffron
The only published study on the relation between IOP and saffron comes from Iran, the world’s largest saffron exporter. Jabbarpoor Bonyadi et al. [rx] studied the effect of a daily oral saffron capsule on IOP in patients with POAG. After a period of 3 weeks, a statistically significant decline in IOP was noted among the study group. They suggested that this hypotensive action is secondary to the antioxidative effect of saffron [rx]
https://youtu.be/ckk7PiODB18
Complications
Complications of glaucoma include
-
Blindness- usually painless
-
Painful blind eye/Absolute glaucoma- OAG predisposes to central retinal venous occlusion, which can give rise to neovascular glaucoma and painful blind eye.
Prevention
These self-care steps can help you detect glaucoma in its early stages, which is important in preventing vision loss or slowing its progress.
- Get regular dilated eye examinations – Regular comprehensive eye exams can help detect glaucoma in its early stages, before significant damage occurs. As a general rule, the American Academy of Ophthalmology recommends having a comprehensive eye exam every five to 10 years if you’re under 40 years old; every two to four years if you’re 40 to 54 years old; every one to three years if you’re 55 to 64 years old; and every one to two years if you’re older than 65. If you’re at risk of glaucoma, you’ll need more frequent screening. Ask your doctor to recommend the right screening schedule for you.
- Know your family’s eye health history – Glaucoma tends to run in families. If you’re at increased risk, you may need more frequent screening.
- Exercise safely – Regular, moderate exercise may help prevent glaucoma by reducing eye pressure. Talk with your doctor about an appropriate exercise program.
- Take prescribed eyedrops regularly – Glaucoma eyedrops can significantly reduce the risk that high eye pressure will progress to glaucoma. To be effective, eyedrops prescribed by your doctor need to be used regularly even if you have no symptoms.
- Wear eye protection – Serious eye injuries can lead to glaucoma. Wear eye protection when using power tools or playing high-speed racket sports in enclosed courts.
References
[bg_collapse view=”button-orange” color=”#4a4949″ expand_text=”Show More” collapse_text=”Show Less” ]
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3484725/
- https://www.ncbi.nlm.nih.gov/books/NBK441887/
- https://www.ncbi.nlm.nih.gov/books/NBK430903/
- https://www.ncbi.nlm.nih.gov/books/NBK367575/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5997592/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5383456/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3700399/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3038509/
- https://www.ncbi.nlm.nih.gov/books/NBK367579/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4562857/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4523637/
- https://www.ncbi.nlm.nih.gov/books/NBK464184/
- https://en.wikipedia.org/wiki/Glaucoma
- https://en.wikipedia.org/wiki/Glaucoma_medication
- https://www.aoa.org/glossary-of-eye-and-vision-conditions/glaucoma
- https://www.medicalnewstoday.com/articles/9710.php
- https://www.medicinenet.com/glaucoma/article.htm
- https://nei.nih.gov/health/glaucoma/glaucoma_facts
- https://www.mayoclinic.org/diseases-conditions/glaucoma/symptoms-causes/syc-20372839
- https://www.webmd.com/eye-health/glaucoma-eyes
- https://www.nhs.uk/conditions/glaucoma/treatments/
[/bg_collapse]